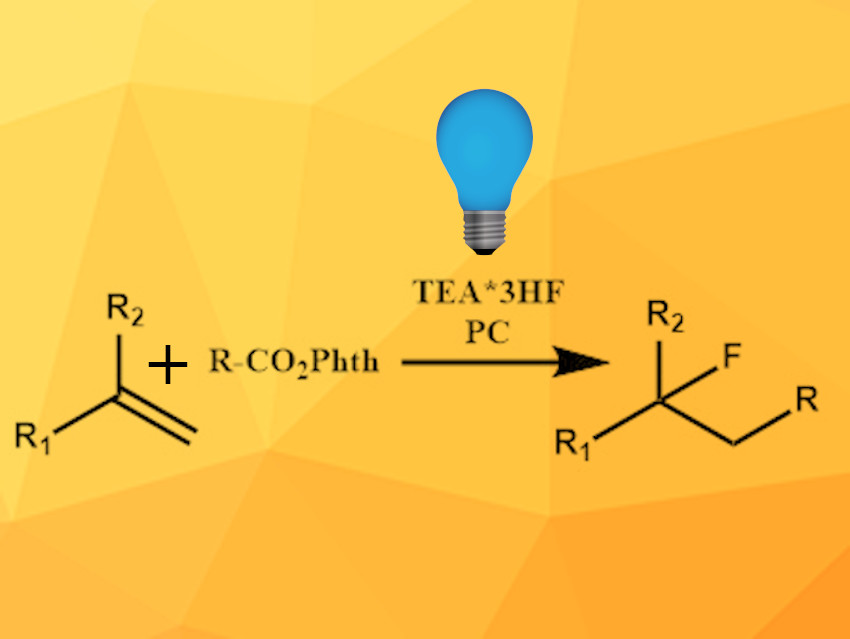
The presence of fluorine atoms alters the properties of molecules, which can be especially important for the synthesis of pharmaceutically active compounds. The incorporation of fluorine into molecules can be more challenging than chlorination or bromination, often requiring expensive reagents or special equipment. The application of photoredox catalysis provides new pathways towards fluorinated organic molecules from simple precursors.
Jaehoon Sim, Chungnam National University, Daejeon, Republic of Korea, and colleagues have developed a photoredox-catalyzed, selective alkylfluorination protocol. A three-component reaction of a phenyl-substituted olefin, an N-hydroxyphthalimide (NHPI) ester as an alkyl radical source, and triethylamine (TEA)·3HF as a fluorine source was catalyzed by the iridium complex Ir(dtbbpy)(ppy)2PF6 (dtbbpy = di-tert-butylbipyridine, ppy = 2-phenylpyridine) under irradiation by a blue LED. The reaction was carried out in dichloromethane (DCM) at room temperature.
Initially, biphenyl-substituted olefins were used as substrates, but many of the corresponding products were unstable and underwent elimination. Styrene-type olefins, however, form bench-stable products. The reaction is compatible with a wide range of styrene derivatives bearing electron-donating and electron-withdrawing substituents in different positions on the aromatic ring. The desired products were isolated in moderate to good yields.
- Photoredox-Catalyzed Oxidative Radical–Polar Crossover Enables the Alkylfluorination of Olefins,
Eunbin Jang, Hoe In Kim, Hye Su Jang, Jaehoon Sim,
J. Org. Chem. 2022.
https://doi.org/10.1021/acs.joc.1c02607