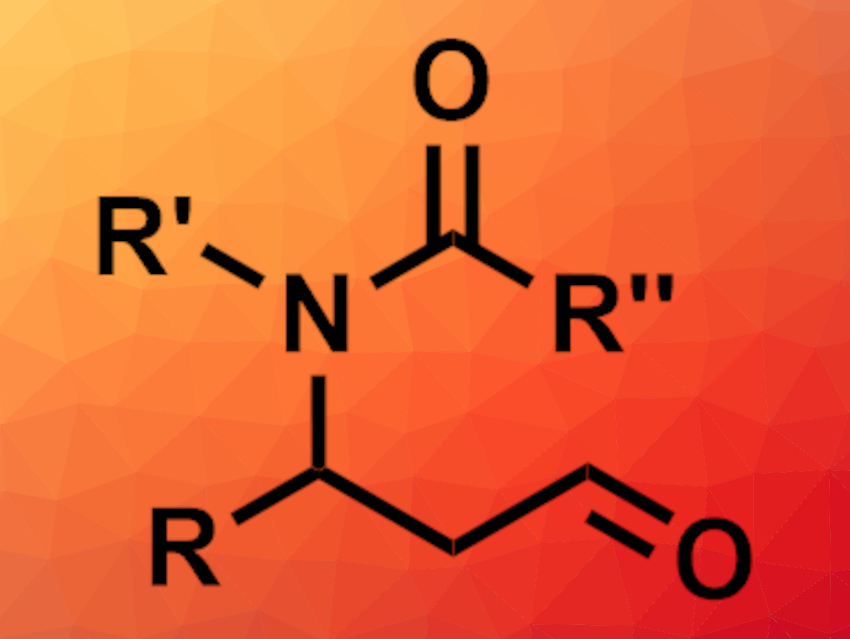
β-Amino aldehydes can be useful intermediates in organic synthesis, e.g., in the preparation of natural products and or bioactive compounds. They can, for example, be synthesized via an anti-Markovnikov oxidation of allylic amines. However, the necessary substrates can be challenging to obtain. Allylic alcohols might be a useful alternative.
Jian-Ping Qu, Nanjing Tech University, China, Yan-Biao Kang, University of Science and Technology of China, Hefei, and colleagues have developed a tandem isomerization/anti-Markovnikov oxidation that transforms linear allylic imidic esters directly to β-amino aldehydes (pictured). The team used Pd(PhCN)2Cl2 as a catalyst, tert-butyl nitrite as a redox cocatalyst, O2 as the only oxidant, and tert-butanol as the solvent. The reactions were performed at room temperature.

The desired β-amino aldehydes were obtained in moderate to good yields. The team found that the use of tert-butanol is important for achieving high aldehyde selectivity. The protocol uses mild conditions and avoids a need for hazardous cocatalysts.
- Direct Synthesis of β-Amino Aldehydes from Linear Allylic Esters Using O2 as the Sole Oxidant,
Shu-Hui Lei, Ya Zhong, Xian-Peng Cai, Qing Huang, Jian-Ping Qu, Yan-Biao Kang,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c03619