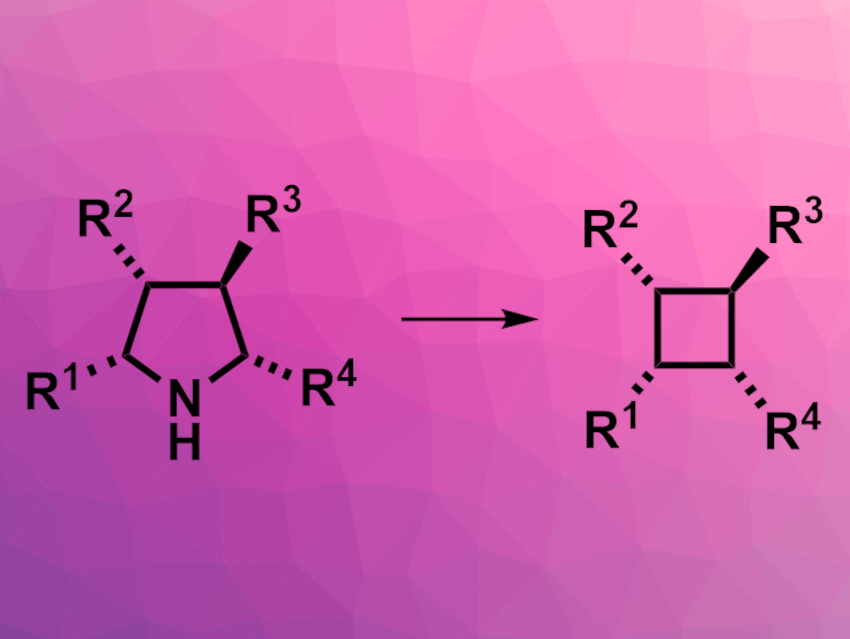
Cyclobutane rings are found, e.g., in some bioactive natural products. They can contain several stereocenters within a fairly limited space. Multisubstituted cyclobutanes can be prepared, for example, using [2+2] cycloadditions, radical cyclizations, or ring contractions.
Andrey P. Antonchick, Max Planck Institute of Molecular Physiology, Dortmund and Technical University Dortmund, both Germany, and Nottingham Trent University, UK, and colleagues have developed a method for the stereoselective synthesis of substituted cyclobutanes from readily accessible pyrrolidines (pictured). The team reacted a variety of polysubstituted pyrrolidine derivatives with hydroxy(tosyloxy)iodobenzene (HTIB) and ammonium carbamate in 2,2,2-trifluoroethanol at 80 °C.
Depending on the substitution pattern of the pyrrolidines, the desired cyclobutanes were obtained in low to good yields. The reaction proceeded in a stereoselective manner. The researchers propose a reaction mechanism that involves an in–situ-generated iodonitrene species that reacts with the pyrrolidine to form a reactive 1,1-diazene. The elimination of N2 then gives a 1,4-biradical, which undergoes cyclization to give the corresponding cyclobutane. The team used the developed method successfully in a concise synthesis of the cyclobutane-containing natural product piperarborenine B.
- Stereoselective Synthesis of Cyclobutanes by Contraction of Pyrrolidines,
Chunngai Hui, Lukas Brieger, Carsten Strohmann, Andrey P. Antonchick,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c10175