Phosphoranides are compounds with a negatively charged tetracoordinated phosphorus atom. Tetrafluorophosphoranide salts of the type M[PF4] are often unstable. However, such compounds could be useful building blocks for the synthesis of phosphoranidometal complexes with unusual structures.
Berthold Hoge, University of Bielefeld, Germany, and colleagues have improved the stability of fluorophosphoranides by replacing two of the fluoride atoms in tetrafluorophosphoranide with electron-withdrawing pentafluoroethyl (C2F5) groups. The team prepared a complex of the stabilized phosphoranide by reacting P(C2F5)2F with silver fluoride, which gives silver difluorobis(pentafluoroethyl)phosphoranide or Ag[P(C2F5)2F2]. A reaction of P(C2F5)2Br with an excess of silver fluoride also gave Ag[P(C2F5)2F2].
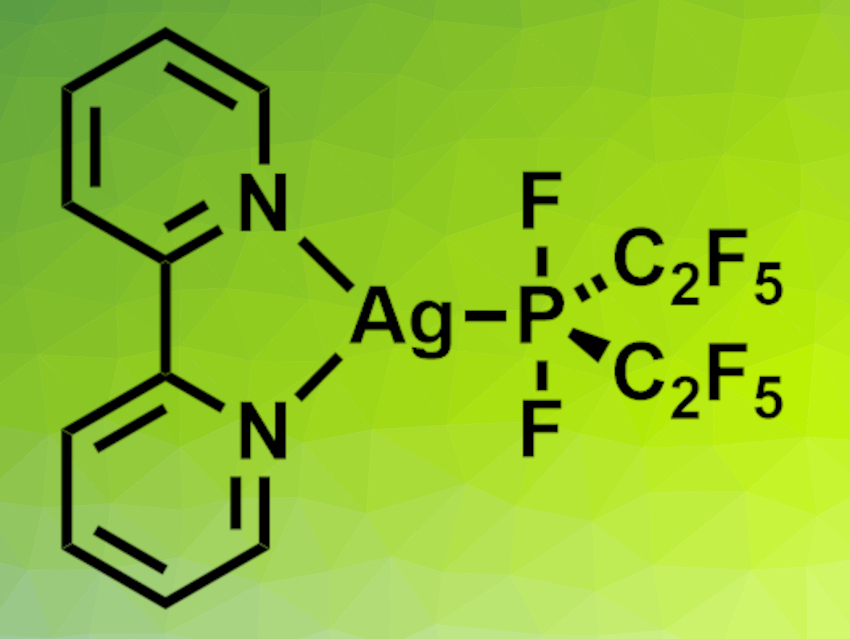
The researchers added 2,2′-bipyridine to obtain the stable, isolable complex [Ag(bipy)(P(C2F5)2F2)] (pictured), which was characterized using X-ray crystallography. Reactions with (NMe4)Cl or CoCl2 in diethylether or MeCN, respectively, gave the bis- and trisphosphoranido complexes [NMe4][Ag(P(C2F5)2F2)2(OEt2)] and [Co(NCMe)6][Ag(P(C2F5)2F2)3]·2 MeCN. According to the researchers, the latter is the first homoleptic phosphoranidometal complex.
- Difluorobis(pentafluoroethyl)phosphoranide: A Promising Building Block for Phosphoranidometal Complexes,
Mira Keßler, Hans-Georg Stammler, Beate Neumann, Gerd-Volker Röschenthaler, Berthold Hoge,
Inorg. Chem. 2021.
https://doi.org/10.1021/acs.inorgchem.1c02307