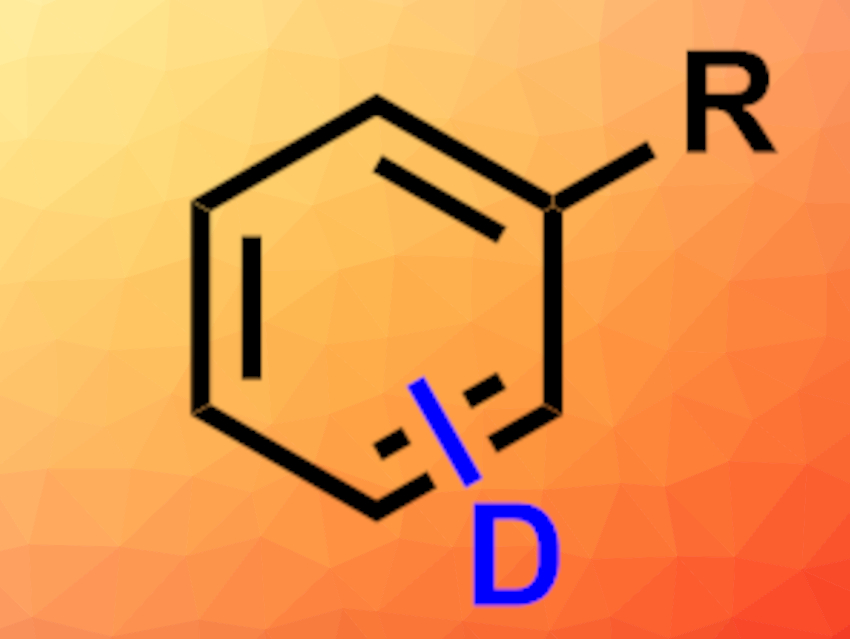
Organic compounds in which hydrogen atoms are replaced with the heavier isotope deuterium are useful, e.g., in investigations of reaction mechanisms, mass spectrometry studies, or drug development. Synthesizing deuterated analogues of organic compounds “from scratch” can be labor-intensive, time-consuming, and costly. Postsynthetic modifications could be an alternative. However, methods for H/D exchange, e.g., at arenes often have problems such as limited substrate scopes.
Manuel van Gemmeren, University of Münster, Germany, and colleagues have developed a palladium-catalyzed, nondirected late-stage deuteration of arenes. The team used Pd(OAc)2 as a catalyst (together with a dual-ligand system) and a mixture of 1,1,1,3,3,3-hexafluoropropan-2-ol (HFIP) and D2O as the solvent and a deuterium source. The researchers observed a high degree of deuterium incorporation, even for complex substrates.
The method can be used for both electron-rich and electron-poor arenes and tolerates a wide range of functional groups. The team used the approach to isotopically label a wide variety of bioactive molecules and related structures. D2O serves as a cheap and convenient deuterium source in the reactions.
- Palladium-Catalyzed Nondirected Late-Stage C–H Deuteration of Arenes,
Mirxan Farizyan, Arup Mondal, Sourjya Mal, Fritz Deufel, Manuel van Gemmeren,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c08233


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
