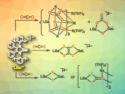
Lithium and sodium salts of the closo-[CB11H12]− anion can be useful as electrolytes for all-solid-state batteries as they provide high ionic conductivity. The closo-carbadodecaborate anions in these salts are weakly coordinating, which is ideal for the movement of cations such as Na+ and Li+. They also have a delocalized electronic structure, making them chemically stable. Existing syntheses of the closo-[CB11H12]− anion can be complex, expensive, and require dangerous reagents that hamper the commercial use of closo-carbadodecaborate anions in electrolytes.
Mark Paskevicius, Curtin University, Perth, Australia, and colleagues have used cost-effective common laboratory reagents to synthesize the closo-[CB11H12]− anion while avoiding reagents like decaborane, NaH, and CF3SiMe3, which can be toxic, flammable, and/or expensive. Instead of using decaborane as a precursor to synthesize the closo-[CB11H12]− anion, the team used nido-[B11H14]– as a precursor. The team deprotonated the anion of Me3NH[B11H14] using NaOH and K2CO3 and reacted it with chloroform to give closo-[CB11H12]−.
Using this method on a gram scale, the team obtained a yield of 40 %, compared with 95 % for previously known approaches. However, the method avoids the use of dangerous substances and the cost of the reagents is significantly lower. The developed method to synthesize Me3NH[CB11H12] could be accessible to any chemist and further the commercial application of closo-[CB11H12]− salts for all-solid-state batteries.
- Synthesis of closo-CB11H12– Salts Using Common Laboratory Reagents,
Amanda Berger, Craig E. Buckley, Mark Paskevicius,
Inorg. Chem. 2021.
https://doi.org/10.1021/acs.inorgchem.1c01896
![Scalable and Safe closo-[CB11H12]– Salt Synthesis](https://www.chemistryviews.org/wp-content/uploads/legacy/common/images/thumbnails/source/17c08d1ba0f.jpg)