Esterification reactions are commonly used transformations in organic synthesis. Often, the carboxylic acids used in these reactions are first converted to activated, reactive species such as acyl chlorides, or harsh conditions are used to achieve ester formation. New mild and efficient protocols for esterification reactions are, thus, of interest.
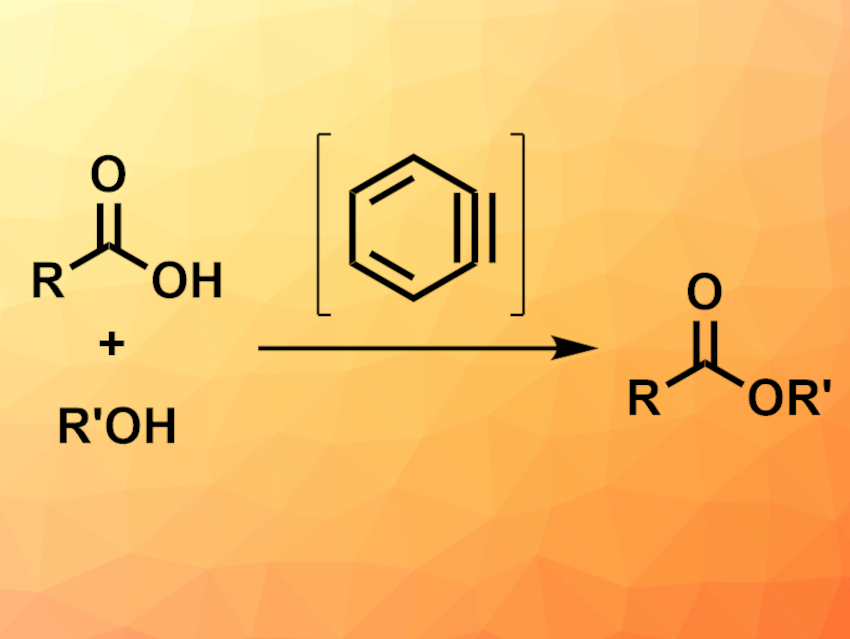
Jiarong Shi, Chongqing University, China, Yang Li, Jilin University, Changchun, China, and Chongqing University, and colleagues have developed a benzyne-mediated esterification of carboxylic acids and alcohols (pictured) that can be performed under mild conditions. The team reacted a variety of aromatic and aliphatic carboxylic acids with different primary or secondary alcohols in the presence of 2-(trimethylsilyl)phenyltriflate as a benzyne precursor, CsF as a fluorine source, and 18-crown-6 or K2CO3 in acetonitrile at 70 °C.
The desired esters were obtained in moderate to good yields. According to the researchers, the approach involves a reaction of benzyne formed in situ with the carboxylic acid to give a phenylester, which then undergoes a transesterification with the alcohol to give the desired product. The protocol can also be used for lactonizations or amidation reactions.
- Benzyne-Mediated Esterification Reaction,
Jinlong Zhao, Jiarong Shi, Yang Li,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c02702



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)