For alkylation reactions at pyridines, regioselectivity can be an issue. To achieve, e.g., a selective C-4 alkylation, substrates usually need to be prefunctionalized at C-4. Alternatively, the C-2 sites can be blocked by a substituent at the pyridine nitrogen that is removed after the reaction to ensure the desired selectivity. However, this can require expensive reagents and/or multiple extra steps.
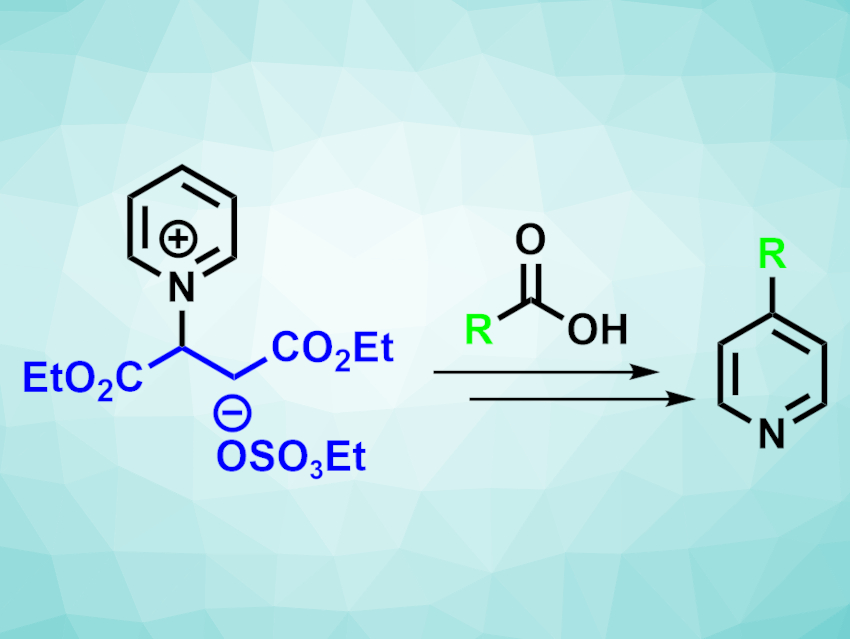
Phil S. Baran, Scripps Research, La Jolla, Ca, USA, and colleagues have developed a selective C-4 alkylation of pyridines that uses a blocking group derived from inexpensive maleic acid that can be introduced in two steps without a need for purification. The team started from pyridine and maleic acid—two commodity chemicals—and reacted them to install a fumarate blocking group at the nitrogen, which was then converted to the corresponding diethyl ester. The resulting pyridinium salt (pictured above on the left) is a stable, easy-to-handle solid.
The pyridinium salt was then used as a substrate in a variety of alkylation reactions under acid-free Minisci conditions. It was reacted with a range of primary, secondary, and tertiary carboxylic acids. The desired C-4-alkylated pyridines were obtained in moderate to good yields. According to the researchers, the developed method is more practical and cost-effective for the synthesis of many of the obtained products than previous approaches.
- Practical and Regioselective Synthesis of C-4-Alkylated Pyridines,
Jin Choi, Gabriele Laudadio, Edouard Godineau, Phil S. Baran,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c05278