Organolithium compounds are important reagents in organic synthesis. Usually, these compounds form aggregates, both in solution and in the solid state. This stabilizes the organolithium compounds and reduces their reactivity. Lewis-basic ligands can break apart these aggregates and form ligand-supported RLi monomers. However, an isolable ligand-supported MeLi monomer had been unknown so far. Previous attempts had resulted in dimers.
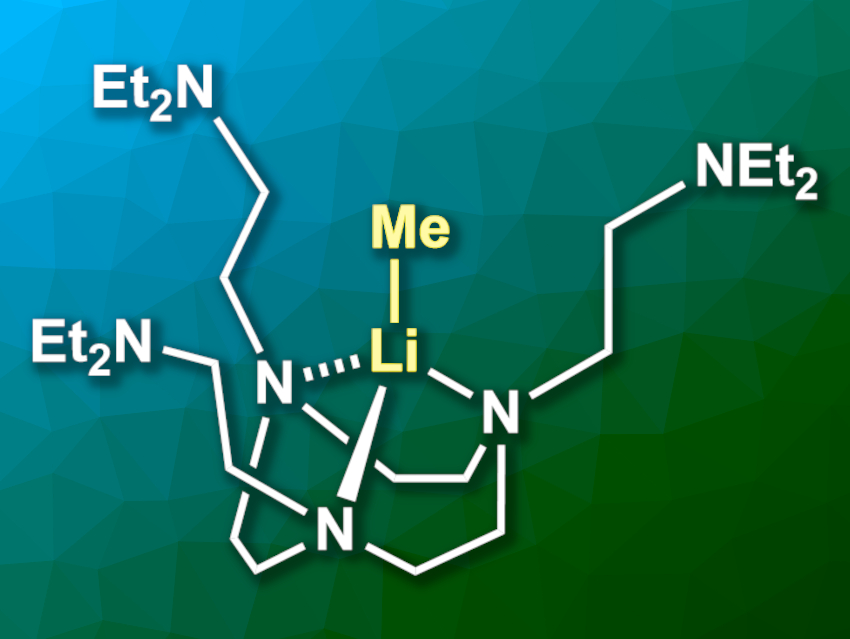
Erli Lu, Newcastle University, Newcastle upon Tyne, UK, and colleagues have synthesized the first isolable monomeric MeLi complex, Li(CH3)(κ3-N,N’,N’-DETAN) (pictured), using the hexadentate ligand N,N’,N’-tris-(2-N-diethylaminoethyl)-1,4,7-triaza-cyclononane (DETAN). The team synthesized the DETAN ligand from 1,4,7-triazacyclononane (TACN) and 2-chloro-N,N-diethylacetamide. The ligand was then reacted with MeLi in a mixture of hexane/Et2O at temperatures of −80 °C to −30 °C.
The desired MeLi monomer was obtained in a yield of 25 % in the form of colorless crystals. It is stable for days at temperatures below –20 °C and decomposes fairly quickly at room temperature. The researchers confirmed the monomeric structure using single-crystal X-ray diffraction. According to the team, reactivity studies of the complex are underway.
- A monomeric methyllithium complex: synthesis and structure,
Nathan Davison, Emanuele Falbo, Paul G. Waddell, Thomas J. Penfold, Erli Lu,
Chem. Commun. 2021.
https://doi.org/10.1039/d1cc01420j