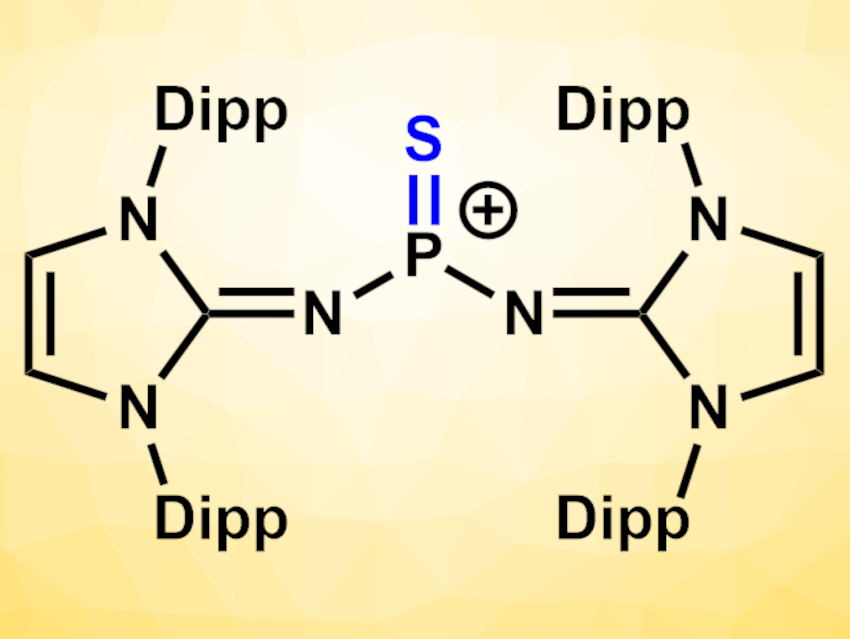
In organic synthesis, carbonyl groups can be transformed to thiocarbonyls using sulfur atom transfer reagents. Generally, P4S10 or the dimeric Lawesson’s reagent (H3C–C6H4–PS2)2 are used for this type of reaction. These reagents are cleaved into reactive dithiophosphoranes of the type RPS2, which then react with the carbonyl compounds. More reactive monomeric species could be useful for thionation reactions.
Fabian Dielmann, University of Münster, Germany, and University of Innsbruck, Austria, and colleagues have synthesized a Lewis base-free, monomeric thiophosphonium species of the type [R2P=S]+ (pictured, Dipp = 2,6-diisopropylphenyl) that can transfer sulfur atoms to both carbonyl and phosphorus compounds. The compound can be considered a more reactive monomeric alternative to Lawesson’s reagent, stabilized by bulky N-heterocyclic imines. The team started from a SiMe3-substituted 1,3-bis(2,6-diisopropylphenyl)imidazolin-2-imine, which was reacted in a stepwise manner with PSCl3 to replace two of the Cl atoms with imine units. In a final step, a chloride anion was removed by treatment with AgOTf or NaBArF24 (OTf− = trifluoromethanesulfonate, BArF24− = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate) to give the corresponding thiophosphonium salts and AgCl or NaCl.
The thiophosphonium ions can react with carbonyl compounds to give thiocarbonyls at room temperature, i.e., under milder conditions than Lawesson’s reagent. They can also undergo chalcogen metathesis reactions with phosphine chalcogenides: Compounds of the type R3P=X (X = O,Se,Te) are converted to R3P=S.
- Lewis base-free thiophosphonium ion: a cationic sulfur atom transfer reagent,
Pawel Löwe, Tim Witteler, Fabian Dielmann,
Chem. Commun. 2021.
https://doi.org/10.1039/d1cc01273h