Reactions that form peptide bonds are important, e.g., for making peptide therapeutics in pharmaceutical chemistry. However, the synthesis of peptide chains usually requires many protection and deprotection steps to ensure that the right functional groups react with each other. This increases the costs and the environmental impact of the processes. Known examples of peptide bond-forming reactions using unprotected amino acids have issues such as racemization, low yields, or limited substrate scopes.
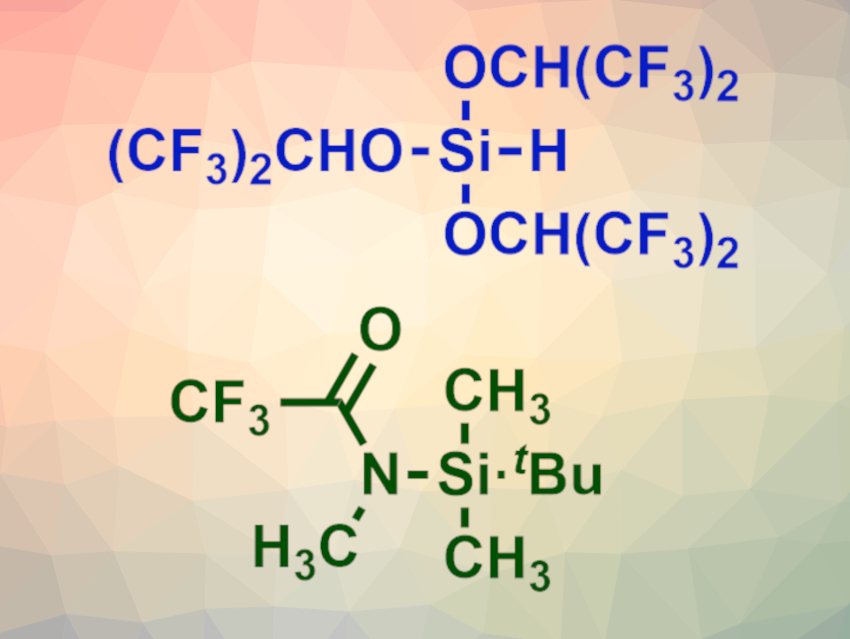
Wataru Muramatsu and Hisashi Yamamoto, Chubu University, Kasugai, Japan, have developed a one-pot peptide bond-forming reaction that is based on two different silylating reagents. The team used HSi[OCH(CF3)2]3 (pictured at the top) to form a silyl ester with the carboxyl group of the amino acid substrate via a chemoselective silylation. Then N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide (MTBSTFA, pictured at the bottom) was added to the reaction mixture to transiently protect the amino group of the substrate. The coupling partner, an amino acid tert-butyl ester, was added last. CsF and imidazole were used as catalysts.
The desired peptide was formed in high yield and without racemization. The reaction is easy to operate and has a broad substrate scope, with a wide variety of unprotected and partially protected amino acids as electrophiles and different amino acid esters as nucleophiles.
- Peptide Bond Formation of Amino Acids by Transient Masking with Silylating Reagents,
Wataru Muramatsu, Hisashi Yamamoto,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c02600