Nitric oxide (NO) is an important signaling molecule with medical applications. Since NO is gaseous and difficult to deliver directly, organic molecules with nitroso (–NO) groups that can release NO in situ are used in pharmaceutical chemistry. Known methods for the nitrosation of organic compounds are hampered by the required harsh conditions, unstable reagents, or limited substrate scopes.
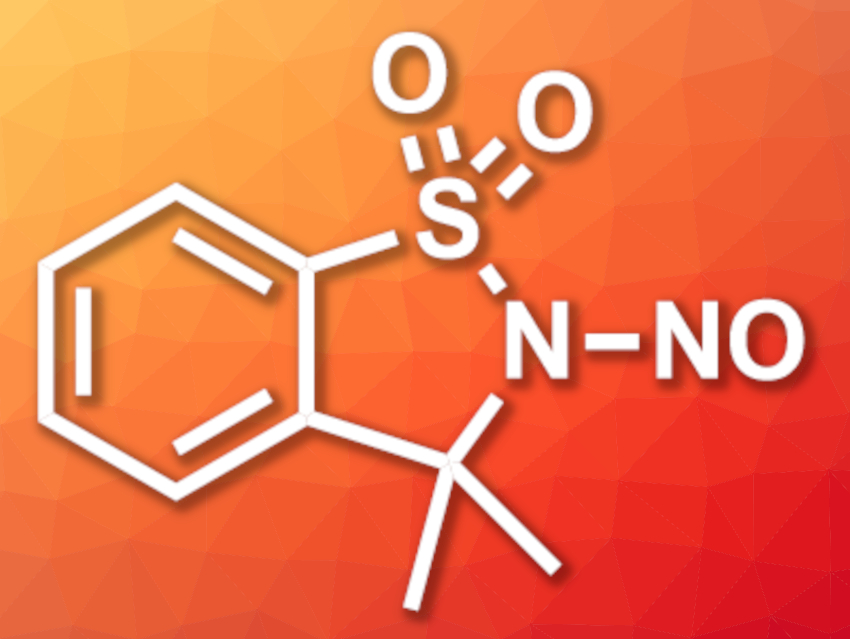
Ryan D. Baxter, University of California, Merced, USA, and colleagues have developed a new bench-stable reagent (3,3-dimethyl-2-nitroso-2,3-dihydrobenzo[d]isothiazole-1,1-dioxide, pictured) for nitrosation reactions that can be used under mild reaction conditions at a broad range of temperatures and in different solvents. The N-nitrososulfonamide-based reagent was prepared on a multigram scale from the artificial sweetener saccharin. Saccharin was first reacted with SOCl2 to give 3-chlorobenzo[d]isothiazole-1,1-dioxide and then methylated using MeLi. Finally, the nitroso group was installed via a reaction with NaNO2.
The reagent can be stored under ambient conditions. It allows the irreversible nitrosation of alkyl alcohols, amines, amides, ureas, and thiols in high yields, under mild conditions, and with good functional group tolerance. After the nitrosation reactions, the resulting sulfonamide can be recovered and converted back to the original reactant by a reaction with NaNO2.
- Versatile New Reagent for Nitrosation under Mild Conditions,
Jordan D. Galloway, Cristian Sarabia, James C. Fettinger, Hrant P. Hratchian, Ryan D. Baxter,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c00637