CO2 capture and utilization (CCU) processes can be useful to mitigate greenhouse gas emissions and provide value-added products at the same time. Efficient methods for the capture of CO2 from air or flue gas and its subsequent conversion to C1 building blocks or fuels could, thus, help to fight climate change and improve the sustainability of the economy. Amines, for example, can be used to capture CO2, but their use also has environmental disadvantages. Natural amino acids, such as L-lysine (Lys), could be an alternative.
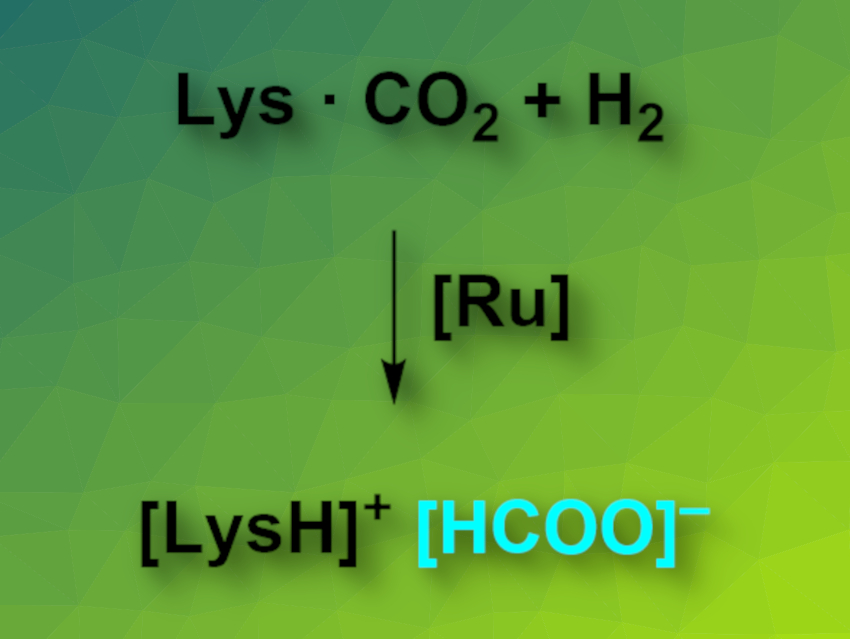
Duo Wei, Henrik Junge, and Matthias Beller, Leibniz Institute for Catalysis, Rostock, Germany, have developed an amino-acid-based CCU system. The reaction system (pictured) uses lysine to capture CO2 and a ruthenium catalyst to produce formates via the hydrogenation of the absorbed carbon dioxide. Lysine can capture CO2 from ambient air to form a carbamate. The researchers were able to perform this reaction on a multigram scale. The team then searched for suitable catalysts for the hydrogenation of CO2 in the presence of lysine. They found that so-called Ru-MACHO complexes, which feature PNP-type pincer ligands, have significant catalytic activity for this reaction.
Using the developed system, CO2 can be captured from ambient air in the form of carbamates and directly hydrogenated to give formate in a one-pot process.
- An amino acid based system for CO2 capture and catalytic utilization to produce formates,
Duo Wei, Henrik Junge, Matthias Beller,
Chem. Sci. 2021.
https://doi.org/10.1039/d1sc00467k