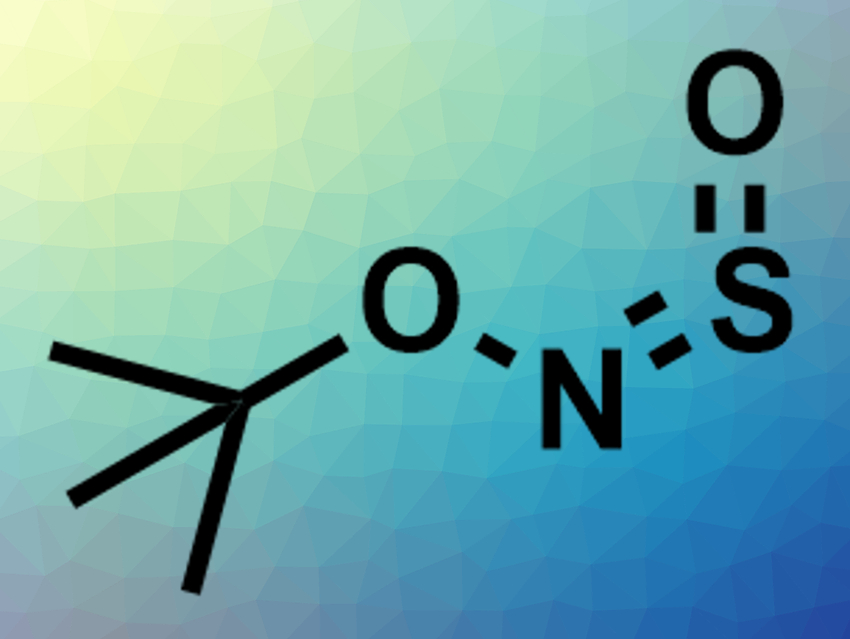
Sulfonamides are found in many pharmaceutically active compounds and several widely used drugs. Primary sulfonamides are also useful intermediates in organic synthesis. Usually, primary sulfonamides are prepared via a reaction of sulfonyl chlorides or similar activated species with ammonia, followed by a deprotection step. However, this synthesis involves harsh conditions and hard-to-handle reagents. Alternative approaches suffer from, e.g., limited substrate scopes or a need for harmful reagents.
Michael C. Willis, University of Oxford, UK, and colleagues have developed a new sulfinylamine reagent, N-sulfinyl-O-(tert-butyl)hydroxylamine (t-BuONSO, pictured), that can be used for the synthesis of primary sulfonamides. The t-BuONSO reagent was prepared on a 15 g scale from commercially available O–tert-butylhydroxylamine hydrochloride via a reaction with thionyl chloride and triethylamine. The reagent is a stable, colorless liquid.
The team then converted a variety of (hetero)aryl or alkyl Grignard reagents or organolithiums to the corresponding primary sulfonamides by reacting them with t-BuONSO in tetrahydrofuran (THF) at –78 °C. The desired products were obtained in good to excellent yields, and the reaction shows good functional group tolerance. Overall, the new reagent allows for a convenient one-step synthesis of primary sulfonamides.
- Primary Sulfonamide Synthesis Using the Sulfinylamine Reagent N-Sulfinyl-O-(tert-butyl)hydroxylamine, t-BuONSO,
Thomas Q. Davies, Michael J. Tilby, David Skolc, Adrian Hall, Michael C. Willis,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c03505