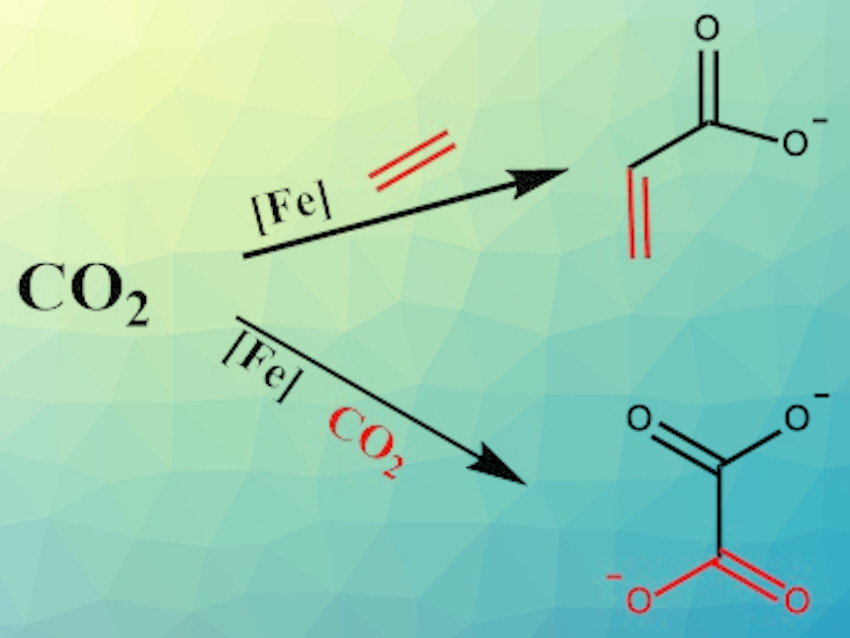
Carbon dioxide is a greenhouse gas but also has the potential to become an important carbon source for value-added products. Processes that, for example, reductively convert CO2 to products such as formic acid or methanol are useful. The direct formation of molecules containing C–C bonds, such as ethylene, acrylate, or oxalate, from CO2 is especially promising because these molecules are important industrial raw materials. However, efficiently converting CO2 to these compounds is more challenging than producing C1-compounds.
Wesley H. Bernskoetter, University of Missouri, Columbia, USA, and colleagues have developed an iron-complex-mediated reductive coupling reaction that can produce oxalate or acrylate from CO2 (pictured). Iron(0)-complexes carrying two 1,2-bis(diethylphosphino)ethane (depe) ligands and either ethylene or CO2 as an additional ligand L were used to mediate the formation of acrylate and oxalate, respectively. The Fe(0)-complex was synthesized via the reduction of the corresponding Fe(II)complex by potassium graphite under nitrogen, which acts as a labile ligand for the Fe(0)-complex.
Replacing the nitrogen ligand by ethylene in situ and subsequent treatment with CO2 and addition of a base led to the formation of acrylate in stoichiometric amounts via an iron-lacton intermediate. Attempts to use the complex catalytically were not successful, however. To produce oxalate, the Fe(depe)2 complex bearing a CO2-ligand was used and treated with KC8 in tetrahydrofuran (THF) under 1.5 atm of CO2 at 45 °C. This reaction gave an excellent oxalate yield of 98 % when stoichiometric amounts of the iron complex were used.
Overall, Fe(depe)2L successfully mediates the formation of acrylate from CO2 and ethylene, as well as the coupling of two CO2 molecules to form oxalate. The reactions cannot be run with catalytic amounts of iron, however. This is presumably due to the complex’s stable 18-electron configuration, which disfavors β-hydride elimination in case of the acrylate formation. For the oxalate formation, there is a competing CO2 disproportionation. Therefore, further studies on more efficient iron complexes are necessary.
- Iron-Mediated C–C Bond Formation via Reductive Coupling with Carbon Dioxide,
Tristan T. Adamson, Steven P. Kelley, Wesley H. Bernskoetter,
Organometallics 2020.
https://doi.org/10.1021/acs.organomet.0c00528

![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

