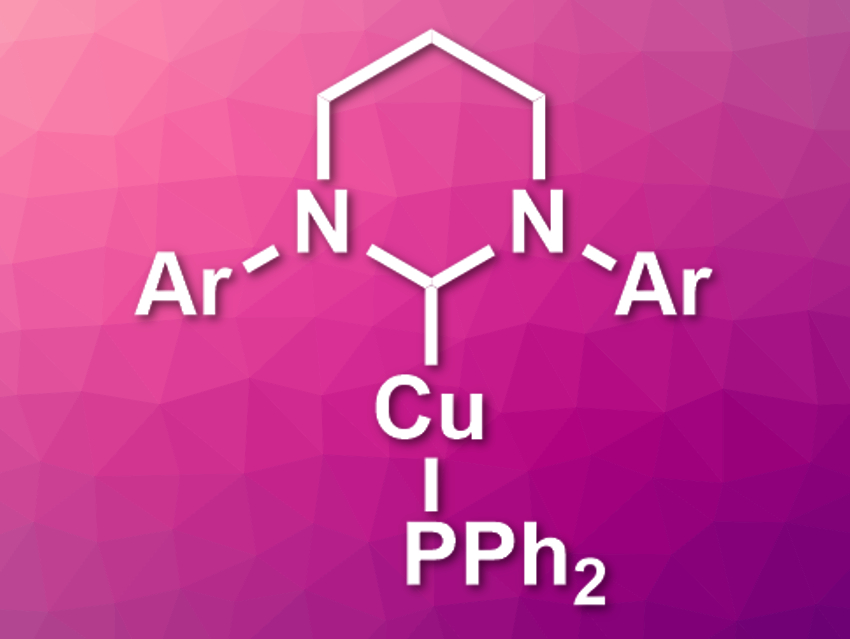
Copper(I) phosphides could be useful for the synthesis of organophosphorus compounds. However, they tend to form oligomers, which reduces their reactivity. Copper complexes with bulky ligands such as sterically demanding N-heterocyclic carbenes (NHCs) could solve this issue, but there are few examples of structurally characterized (NHC)CuPR2 complexes.
David J. Liptrot, Mary F. Mahon, University of Bath, UK, and colleagues have synthesized several N-heterocyclic carbene-supported copper diphenylphosphides, including the first ring-expanded NHC-copper(I) phosphides (pictured). The team synthesized the copper diphenylphosphides from the corresponding (NHC)CuOtBu complexes by reacting them with Ph2PSiMe3 in benzene. The NHCs used were two five-membered NHCs with either 2,6-diisopropylphenyl (Dipp) or mesityl (Mes) substituents and two six-membered NHCs (see picture, Ar = Dipp or Mes).
The resulting complexes were fully characterized using X-ray crystallography and NMR spectroscopy. They exist as monomers or dimers in the solid state and in solution, depending on the steric bulk of the NHC ligand. The team found that the NHC-supported copper diphenylphosphides can be used as highly active and selective precatalysts for the hydrophosphination of isocyanates with Ph2PH.
- The First Ring-Expanded NHC-Copper(I) Phosphides as Catalysts in the Highly Selective Hydrophosphination of Isocyanates,
Thomas Mardale Horsley Downie, Jonathan W. Hall, Thomas P. Collier Finn, David Jonathan Liptrot, John P. Lowe, Mary F. Mahon, Claire L. McMullin, Michael K. Whittlesey,
Chem. Commun. 2020.
https://doi.org/10.1039/d0cc05694d