Cleaving the strong triple bond in N2 is challenging. However, the fixation of dinitrogen is important both in nature and in the chemical industry, where the Haber–Bosch process is used on a huge scale to convert N2 to ammonia. While this industrial process uses an iron-based catalyst and harsh conditions, biological systems use multimetallic clusters for dinitrogen fixation under ambient conditions. Multimetallic clusters with, e.g., uranium might be useful for N2 reduction in the laboratory under mild conditions. However, the complete cleavage of N2 in a six-electron reduction by multimetallic species containing uranium and transition metals had not been reported so far.
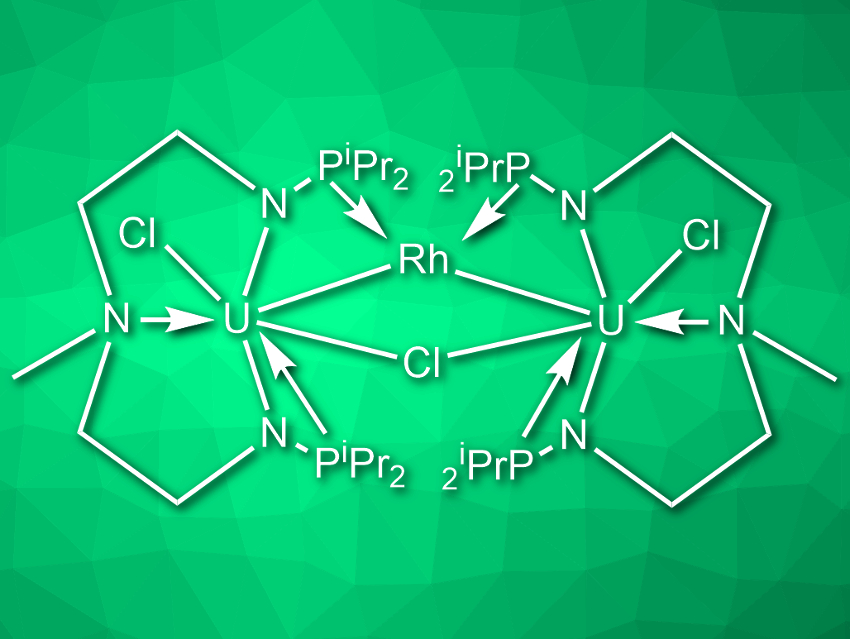
Laurent Maron, Université Paul Sabatier, Toulouse, France, Congqing Zhu, Nanjing University, China, and colleagues have developed the first multimetallic uranium–rhodium cluster that cleaves N2 to two nitrides in the presence of a potassium-based reducing agent at ambient conditions. The cluster (pictured) was synthesized by first reacting uranium tetrachloride with the ligand CH3N(CH2CH2NHPiPr2)2 in the presence of nBuLi in tetrahydrofuran (THF). The resulting uranium complex was then reacted with [RhCl(COD)]2 (COD = cyclooctadiene) in toluene and finally reduced with potassium graphite (KC8) in THF.
When the cluster is reacted with KC8 under a nitrogen atmosphere, it cleaves N2 to form a complex with the formula [{U2[N(CH3)(CH2CH2NPiPr2)2]2(Rh)(μ-N)}2]. The team verified that the nitrogen in the two nitride ligands stems from N2 by isotopic labeling. Under acidic conditions, the nitride-containing complex releases ammonium ions. Overall, the uranium–rhodium cluster performs the binding, activation, and complete six-electron reductive cleavage of N2.
- Dinitrogen Cleavage by a Heterometallic Cluster Featuring Multiple Uranium–Rhodium Bonds,
Xiaoqing Xin, Iskander Douair, Yue Zhao, Shuao Wang, Laurent Maron, Congqing Zhu,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c05788