The hydrogenation of esters is a useful reaction that is often promoted by Ru-, Fe-, or Mn-based catalysts and usually involves gaseous hydrogen as a reactant. Using a liquid hydrogen source, such as an alcohol, instead of H2 in a transfer hydrogenation can be a more practical alternative. However, such transfer hydrogenations of esters are fairly rare so far, and existing examples need expensive metals or high catalyst loadings.
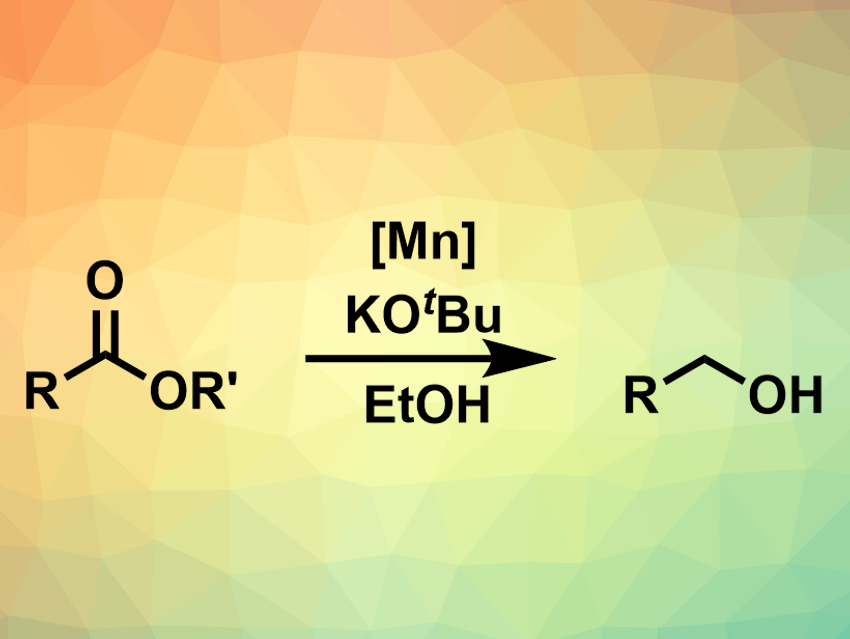
Matthew L. Clarke and colleagues, University of St Andrews, UK, have developed a transfer hydrogenation of esters (pictured above) using ethanol as both hydrogen-transfer agent and low-cost solvent, catalyzed by a low loading of a sustainable manganese catalyst (pictured on the right, Ar = 3,5-Me2-4-OMeC6H2). The team used 1 mol% of the catalyst together with KOtBu as a base to convert a variety of ethyl- or methyl esters to the corresponding primary alcohols.
to convert a variety of ethyl- or methyl esters to the corresponding primary alcohols.
The desired products were obtained in good yields. The developed approach can also be used for one-pot conversions of carboxylic acids to alcohols: The acid is first converted to the corresponding ethyl ester using N,N‘-diisopropylcarbodiimide (DIC) and dimethylaminopyridine (DMAP) in EtOH and then hydrogenated to give the alcohol.
- Manganese-catalysed transfer hydrogenation of esters,
Conor L. Oates, Magnus B. Widegren, Matthew L. Clarke,
Chem. Commun. 2020.
https://doi.org/10.1039/d0cc02598d