Conventional cross-coupling reactions usually take place between an electrophile and an organometallic reagent. In cross-electrophile couplings, in contrast, the coupling partners are both electrophiles. Cross-electrophile couplings generally use alkyl bromides, -iodides, or -sulfonates as substrates, while simple alkyl chlorides are almost never used. Alkyl chlorides could be low-cost, low-toxicity, stable alternatives to the commonly used substrate types. However, their stability also makes them hard to activate using common nickel or photoredox catalysis approaches.
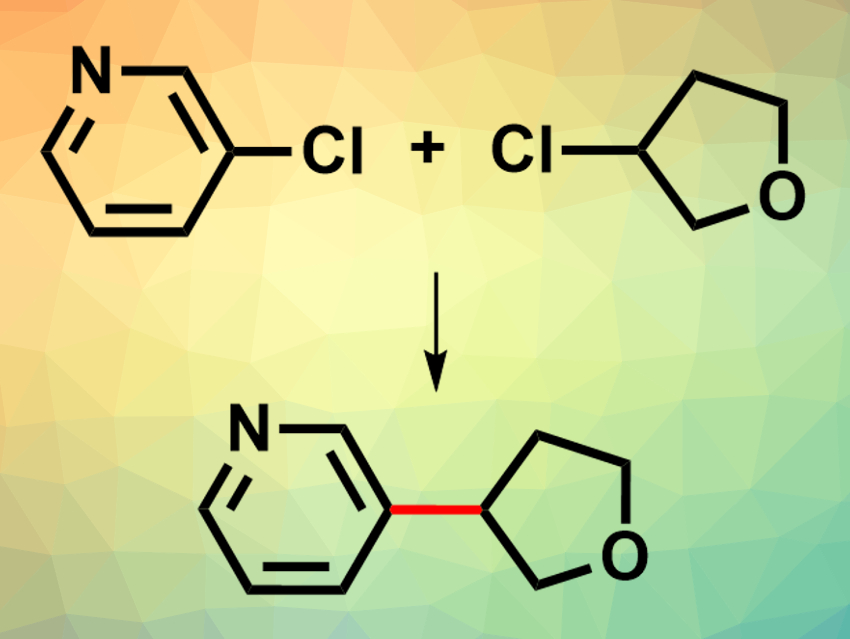
David W. C. MacMillan, Princeton University, NJ, USA, and colleagues have developed the first cross-electrophile coupling of unactivated alkyl chlorides and aryl chlorides (example reaction pictured). The team designed a new organosilane reagent, whose properties are specifically tuned to allow chlorine abstraction from alkyl chlorides, TMS3SiNH(ad) (ad = adamantyl). This reagent was used together with a nickel catalyst (NiCl2·BIm, BIm = 2,2′-biimidazole), an iridium photocatalyst ([Ir(ppy)2(dtbbpy)]PF6; ppy = 2-phenylpyridine, dtbbpy = 4,4′-bis(tert-butyl)-2,2′-bipyridine), and 1,1,3,3-tetramethylguanidine (TMG) as base under visible-light irradiation using a blue LED at 55 °C.
Under these conditions, the researchers coupled a range of unactivated alkyl chlorides with a variety of (hetero)aryl chlorides. The desired coupling products were obtained in good yields. The reaction tolerates electrophilic functional groups such as esters, nitriles, and ketones on the alkyl chloride partner. It works well with both electron-rich and electron-deficient chlorobenzene derivatives, as well as with several heteroarenes, such as chloropyridines or -pyrimidines. The approach can, for example, be used for the synthesis of several pharmaceutically active compounds.
- Cross-Electrophile Coupling of Unactivated Alkyl Chlorides,
Holt A. Sakai, Wei Liu, Chi “Chip” Le, David W. C. MacMillan,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c04812