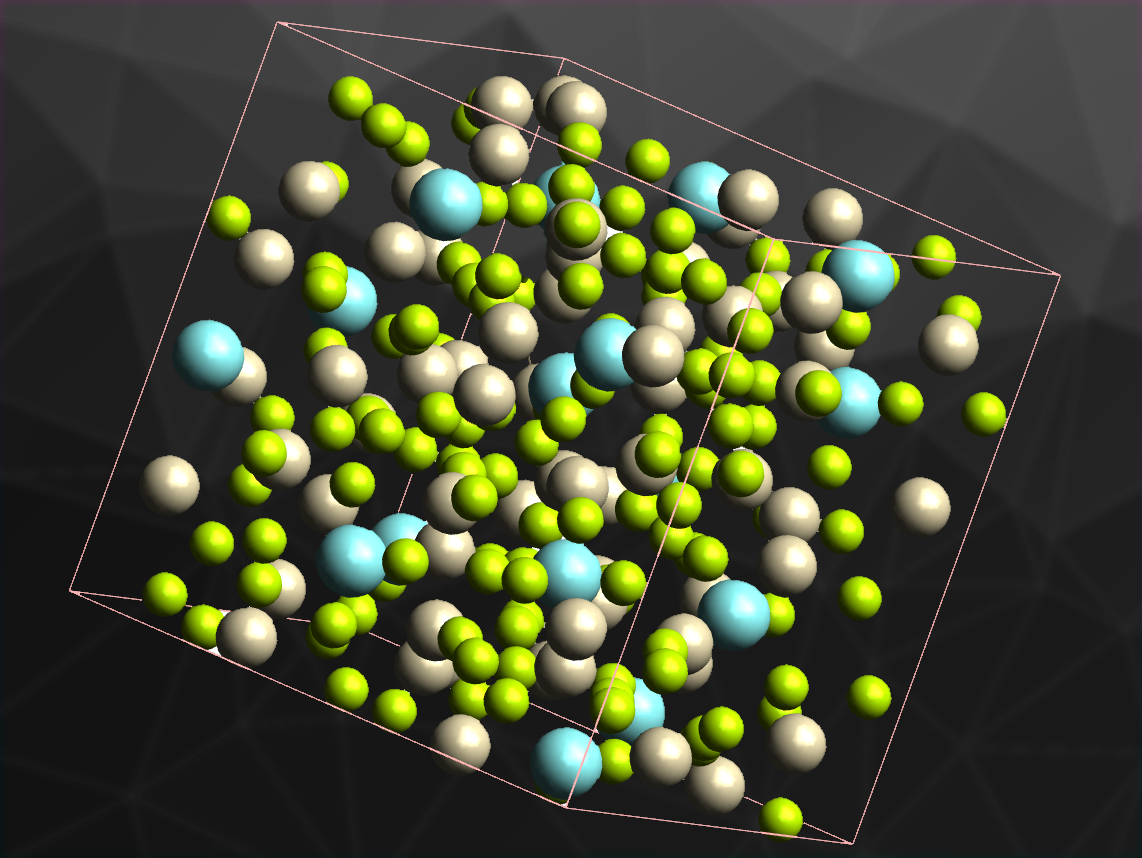
Intermetallic compounds can have very complicated structures with large unit cells. Compounds with particularly large unit cells (more than about 100 atoms) are called complex intermetallic compounds. They often have disordered structures, which could be caused by a low concentration of valence electrons and the resulting multicenter bonds, e.g., in intermetallic compounds with a significant amount of beryllium. However, there is scarce information on ternary intermetallic compounds consisting of Be and other metals, at least in part due to the high toxicity of Be.
Andreas Leithe-Jasper and colleagues, Max Planck Institute for Chemical Physics of Solids, Dresden, Germany, have discovered a new beryllium-rich ternary intermetallic compound, Y4Be33Pt16. The compound was prepared from yttrium pieces, platinum foil, and beryllium sheets by arc melting. X-ray diffraction showed that it crystallizes in the non-centrosymmetric space group I43d.
The Y atoms are located in a cage-like, polyhedral structure consisting of 20 surrounding atoms. Calculations using the QTAIM (Quantum Theory of Atoms In Molecules) method indicate an ionic interaction of the “caged” yttrium with the rest of the framework. The compound has only about 1.4 valence electrons per atom, which leads to multicenter interactions within the Be–Pt framework. This was confirmed by the QTAIM calculations. In addition to these interesting electronic and structural properties, Y4Be33Pt16 becomes superconducting at temperatures below 0.9 K.
- Y4Be33Pt16 – a non-centrosymmetric cage superconductor with multi-centre bonding in the framework,
Alfred Amon, Eteri Svanidze, Yurii Prots, Michael Nicklas, Ulrich Burkhardt, Alim Ormeci, Andreas Leithe-Jasper, Yuri Grin,
Dalton Trans. 2020.
https://doi.org/10.1039/d0dt01374a