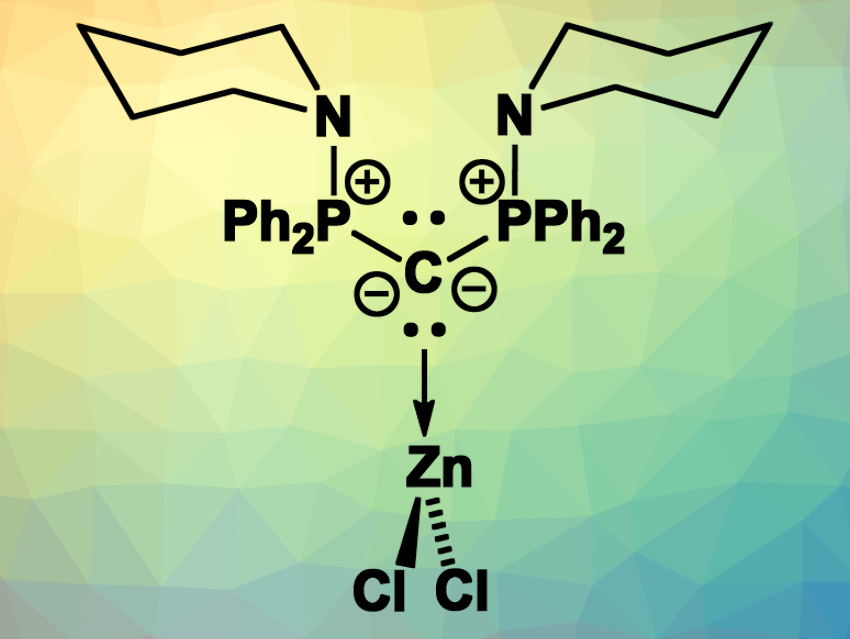
Carbodiphosphoranes (CDPs) are carbon(0) compounds with two PR3 ligands. In these compounds, the C–P-bonds are based on P→C donor–acceptor interactions. As a result, the carbon atom retains two lone pairs of electrons. This unusual bonding situation makes CDPs highly basic and allows them to be used as strong donor ligands, e.g., in transition-metal complexes.
Viktoria H. Gessner, Ruhr University Bochum, Germany, and colleagues have synthesized a diamino-substituted carbodiphosphorane and investigated its coordination chemistry. The team prepared the CDP starting from 1,1-bis(diphenylphosphino)methane (dppm), which was first brominated and then reacted with piperidine to introduce the desired amino groups. This led to the monoprotonated precursor of the diamino-CDP, which was deprotonated using potassium hexamethyldisilazide (KHMDS) to give the free diamino-CDP.
The team investigated the donor strength of the synthesized CDP and found that it is a very strong donor at the carbon atom, with a much weaker donor character at the nitrogen atoms. This led to an unusual zinc complex: The CDP forms a complex with ZnCl2 (pictured) in which the Zn is coordinated at the CDP’s carbon in a trigonal planar coordination environment. Usually, Zn highly favors four-coordinate, tetrahedral structures and binds strongly to N-donors. According to the researchers, no other trigonal planar L·ZnCl2 complex had been reported so far.
- A diamino-substituted carbodiphosphorane as strong C-donor and weak N-donor: isolation of monomeric trigonal-planar L·ZnCl2,
Alexander Kroll, Henning Steinert, Lennart T. Scharf, Thorsten Scherpf, Bert Mallick, Viktoria H. Gessner,
Chem. Commun. 2020.
https://doi.org/10.1039/d0cc02496a