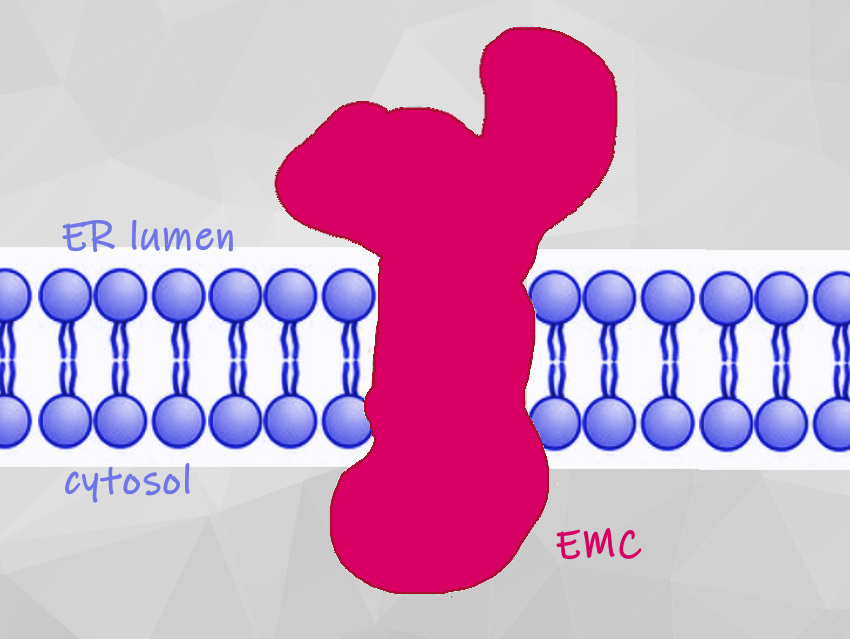
Membrane proteins carry out functions such as regulating blood pressure and coordinating the immune responses. They are manufactured inside the cell and transported to the endoplasmic reticulum (ER). There, they are inserted into the membrane, where they carry out myriad functions. For many membrane proteins, this insertion process relies on a complicated structure known as the ER membrane protein complex (EMC).
Viruses (potentially including SARS-CoV-2) use an EMC to construct and transport some of their viral proteins in an infected organism. Thus, understanding how the EMC works is an important part of understanding basic cellular biology and how to inhibit diseases.
Tino Pleiner, Giovani Pinton Tomaleri, Kurt Januszyk, and colleagues, California Institute of Technology (CalTech), Pasadena, CA, USA, have used single-particle cryo-electron microscopy to determine the structure of the human EMC in a lipid nanodisc. This allowed the team to build a nearly complete atomic model of the EMC.
The new structural model shows that the EMC has nine different essential subunits (EMC1 to EMC8 and EMC10) and how they come together. The EMC helps to guide and insert proteins into the cell membrane, and it probably helps to ensure that they fold and assemble correctly. The team used structure-guided mutagenesis to demonstrate that substrate insertion requires a methionine-rich cytosolic loop and occurs via an enclosed hydrophilic vestibule within the membrane formed by the subunits EMC3 and EMC6. The team thinks that the EMC uses local membrane thinning and a positively charged patch to decrease the energetic barrier for insertion into the bilayer.
The functions of some of the subunits are still not understood. The researchers want to design experiments to investigate them.
- Structural basis for membrane insertion by the human ER membrane protein complex,
Tino Pleiner, Giovani Pinton Tomaleri, Kurt Januszyk, Alison J. Inglis, Masami Hazu, Rebecca M. Voorhees,
Science 2020.
https://doi.org/10.1126/science.abb5008