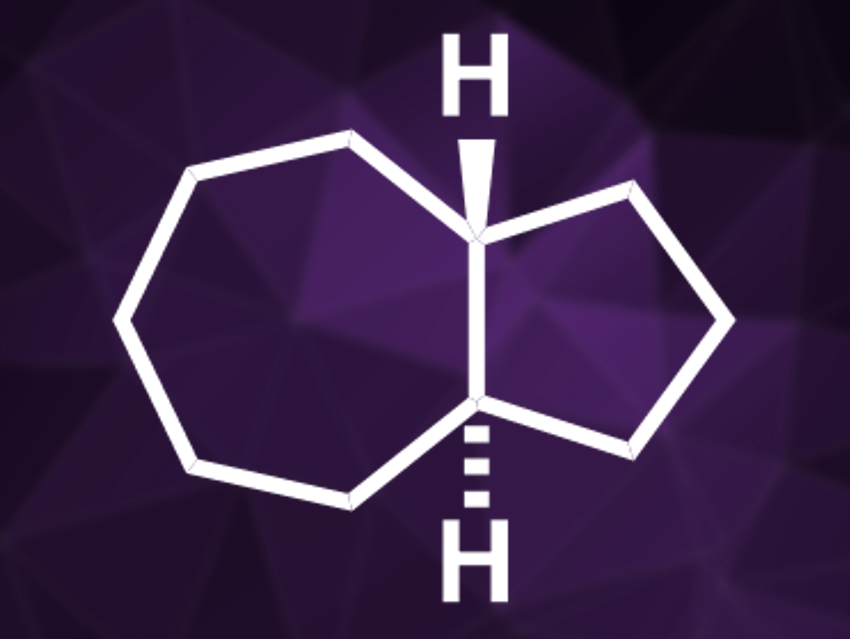
Perhydroazulenes (example pictured) are structural motifs commonly found in terpene natural products. These natural products have been studied extensively, in contrast to the simple hydrocarbon parent compounds. Trans-perhydroazulene (pictured), for example, is chiral, but it had not been prepared in enantiopure form and its absolute configuration had not been determined so far. Finding the absolute configuration of simple alkanes can be challenging because commonly used approaches for this require either heavy atoms or functional groups such as alcohols or amines.
Peter R. Schreiner and colleagues, University of Gießen, Germany, have determined the absolute configuration of trans-perhydroazulene. To achieve this, the team synthesized enantiomerically pure trans-perhydroazulene for the first time. They started from cycloheptanone, which was converted to racemic trans-cycloheptane-1,2-dicarboxylic acid. This racemate was subjected to a chiral resolution by converting it to a diquinine salt, and 99 % enantiopurity was achieved after five recrystallizations. The salt was converted back to the free dicarboxylic acid, which was transformed into the desired, bicyclic trans-perhydroazulene.
With the enantiopure compound in hand, the team used vibrational circular dichroism (VCD) and optical rotatory dispersion (ORD) spectroscopy to assign the absolute configuration. With the help of computed optical rotations and spectra, both methods assigned the absolute configuration of (+)-trans-perhydroazulene as (R,R). The structure of the diquinine salt intermediate, which was determined by X-ray crystallographic analysis, provided further evidence for this assignment based on the known absolute configuration of quinine.
- Absolute Configuration of trans-Perhydroazulene,
Fumito Saito, Dennis Gerbig, Jonathan Becker, Peter R. Schreiner,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c01184