Carbon monoxide is an important raw material for a wide range of industrial processes. It is mainly produced by methane reforming or coal gasification processes. Replacing these fossil-based processes with pathways that use CO2 as a feedstock would be desirable from an environmental point of view. CO2 can, for example, be reduced to CO using transition-metal catalysis. Due to its abundance and low toxicity, iron could be a suitable choice for such reactions.
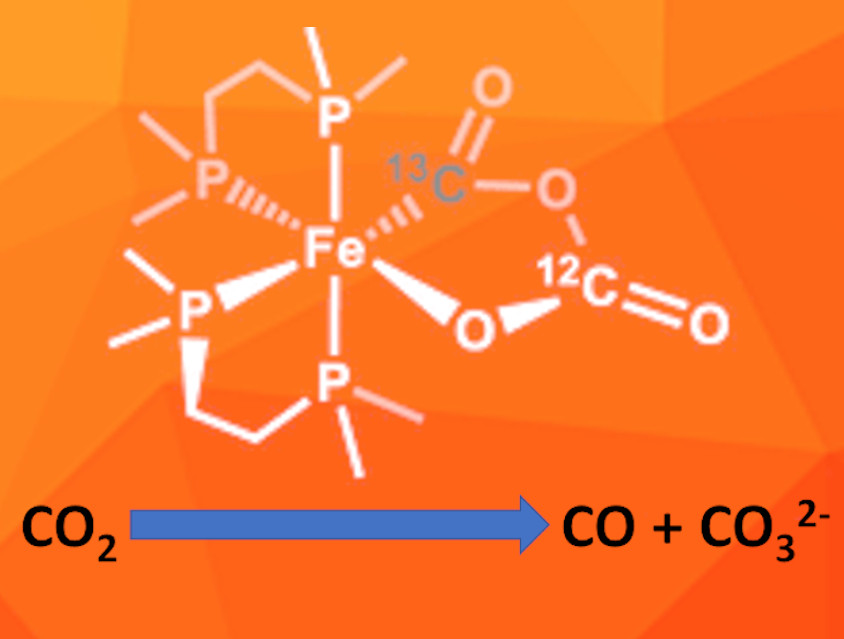
Leslie D. Field, University of New South Wales, Sydney, Australia, and colleagues have used the iron(0) complex [Fe(N2)(dmpe)2] (dmpe=1,1-bis(dimethylphosphino)ethane) for the reductive disproportionation of CO2 to CO and CO32-. They performed a detailed study of the reaction mechanism. The iron complex first reacts with one equivalent of CO2, which replaces the dinitrogen ligand and forms an η2(C,O)-CO2 complex. Then a second CO2 molecule reacts with the complex and forms a metallacycle (pictured) via reductive coupling. The team used 13C-labelled CO2 to differentiate between the CO2 molecules added during the first and second reaction step and confirmed this sequence.
The metallacycle was isolated and characterized by IR- and 13C-NMR-spectroscopy. According to the researchers, it is the first metalo-2,4-dioxolane-3,5-dione containing an iron center. It gradually breaks down, releasing CO and leaving the carbonate complex cis-[Fe(CO3-κ2O,O)(dmpe)2]. This indicates that it could be a key intermediate in the metal-mediated reductive disproportionation of CO2.
- Fe(0)-Mediated Reductive Disproportionation of CO2,
Peter M. Jurd, Hsiu L. Li, Mohan Bhadbhade, Leslie D. Field,
Organometallics 2020.
https://doi.org/10.1021/acs.organomet.0c00175