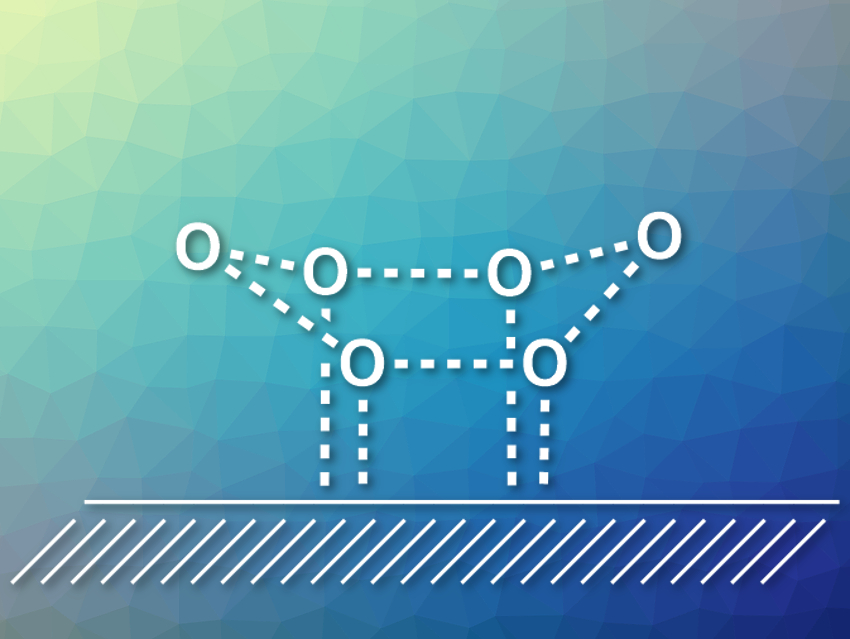
Water can be adsorbed on almost all surfaces. Understanding water/solid interfaces is important in many fields of research and for a variety of applications. Studies of these systems can also provide a better understanding of hydrogen bonding between water molecules and the structure of water clusters. Hydrophobic metal surfaces, for example, are useful for these investigations, but determining the structures that water forms on these surfaces is challenging.
Xin Xu, Fudan University, Shanghai, China, and colleagues have investigated the structure of a water hexamer on Cu(111) surfaces. Water hexamers form a cyclic configuration on such surfaces, which had previously been found using scanning tunneling microscopy (STM). However, its most stable configuration (flat, chair-like, or boat-like) had not been confirmed so far.
The team used theoretical simulations to obtain idealized STM images for all three proposed configurations. They found that the simulated image for a boat-like configuration (pictured, hydrogen atoms omitted) most closely resembles the experimental images. The team also calculated the adsorption energies for all three conformations with high-level quantum-chemical methods and found that the boat configuration is the global energy minimum.
- Identification of Water Hexamer on Cu(111) Surfaces,
Sai Duan, Igor Ying Zhang, Zhen Xie, Xin Xu,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c01549