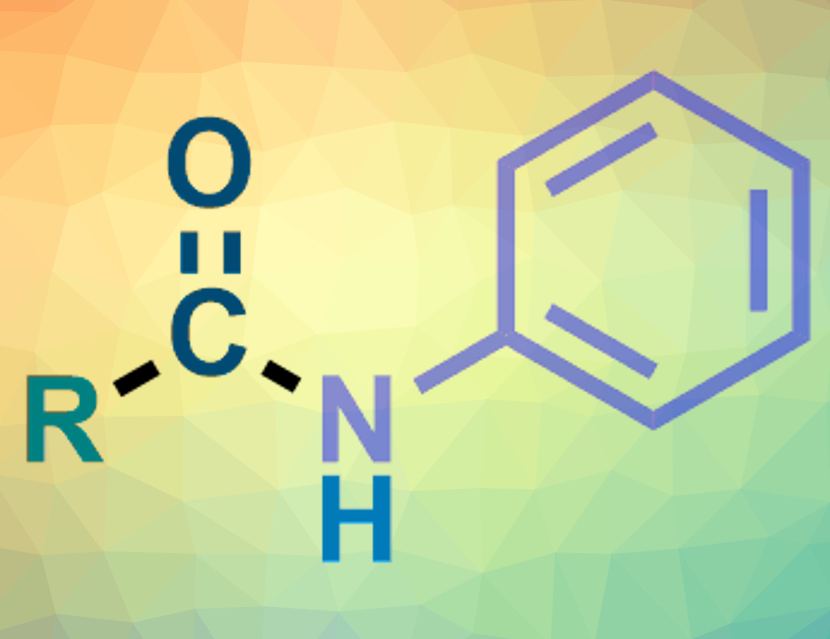
Amides are an important class of organic compounds. N-Aryl amides are usually made from anilines via an aminocarbonylation of aryl halides under a CO atmosphere. Nitroarenes could replace anilines in these reactions when they are used under reductive conditions. However, there are only a few examples of successful reductive aminocarbonylations using nitroarenes.
Siling Zhao and Neal P. Mankad, University of Illinois at Chicago, USA, have developed a copper-catalyzed reductive aminocarbonylation of alkyl iodides under CO using nitroarenes. The team used a copper chloro complex with an N-heterocyclic carbene (NHC) ligand as the catalyst. The NHC with the best performance has two chloro- and two 4-methoxy-2,6-diphenylmethyl substituents. NaOH was used as a base, PhSiH3 as a reductant, and 1,4-dioxane as the solvent. The reaction was performed at 70 °C under 5 atm of CO.
The desired N-aryl alkylamides (pictured) were obtained in moderate to good yields. The team used a variety of primary, secondary, and tertiary alkyl iodides as substrates. The single copper catalyst promotes both the reduction of the nitroarene to give an aniline intermediate and the carbonylation of the alkyl iodide to give an intermediate acyl iodide. These two intermediates can then react to give the amide product.
- Synergistic Copper-Catalyzed Reductive Aminocarbonylation of Alkyl Iodides with Nitroarenes,
Siling Zhao, Neal P. Mankad,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b04092