Organofluorine compounds are often useful, e.g., in medicinal chemistry. There are several methods to synthesize aromatic compounds with fluoroalkyl substituents, while paths to fluoroalkylated alkenes are less well studied. The available methods generally only introduce the fluoroalkyl group into the alkene. Combining this transformation with the introduction of a second useful functional group could be a step-economic way to synthesize complex organic molecules.
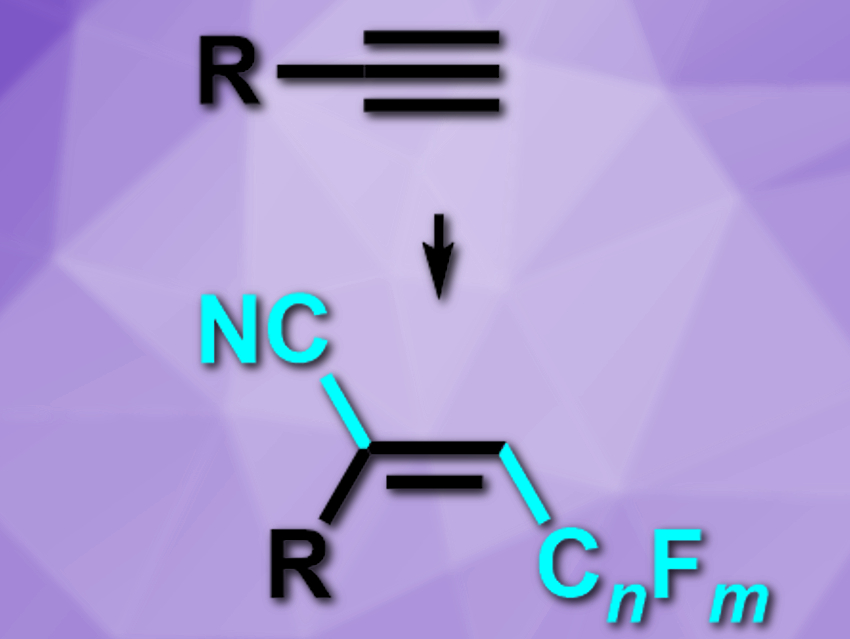
Hongli Bao, University of the Chinese Academy of Sciences, Fujian and Beijing, and colleagues have developed a copper-catalyzed radical cyanoperfluoroalkylation of alkynes to give doubly functionalized alkenes (pictured). The team used a three-component reaction between alkynes, perfluoroalkyl iodides, and trimethylsilyl cyanide. The reaction was catalyzed by Cu(CH3CN)4BF4, together with 2,2′:6′,2”-terpyridine as a ligand and lauroyl peroxide (LPO) as a radical initiator.
The desired products were obtained in generally good yields and the reaction has good functional group tolerance. The cyano group can be further transformed into other functional groups, which makes the reaction’s products useful intermediates for organic synthesis.
- Copper(I)-Catalyzed Cyanoperfluoroalkylation of Alkynes,
Muhammad Israr, Haigen Xiong, Yajun Li, Hongli Bao,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b02648


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
