N-heterocyclic carbenes (NHCs) are commonly used ligands in transition-metal catalysis. For enantioselective transformations, chiral NHCs can be used. The subgroup of cyclic (alkyl)(amino)carbenes (CAACs) has better σ-donating and π-accepting properties than NHCs and can, thus, be used for the improvement of some catalytic processes.
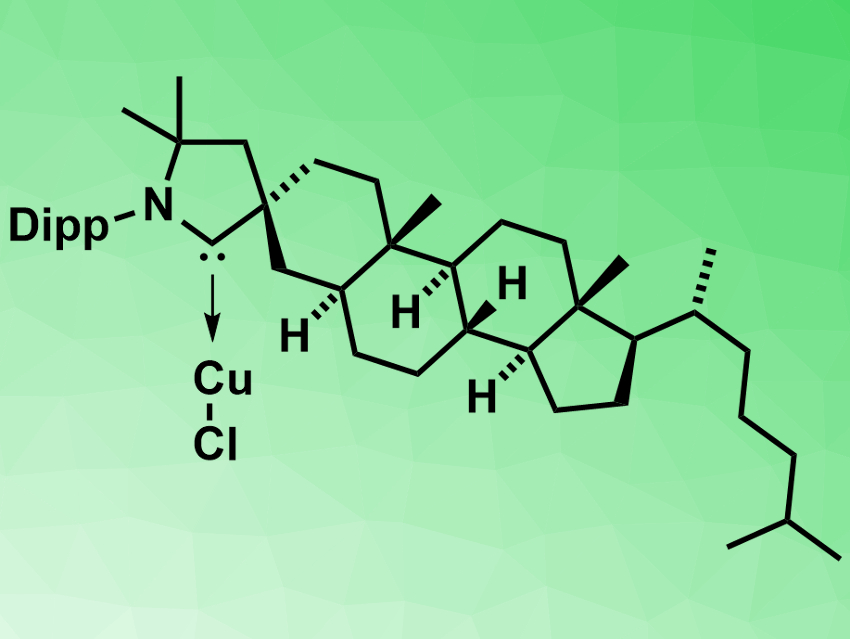
Guy Bertrand, Rodolphe Jazzar, both University of California San Diego, La Jolla, USA, Marc Mauduit, University of Rennes, France, and colleagues have developed a new cholesterol-derived cyclic (alkyl)(amino)carbene (CholestCAAC). They chose the steroid backbone because of its rigidity and availability from the chiral pool. An iminium precursor could be prepared from a coprostanol-derived steroid in 63 % yield over six steps. It was converted into the copper complex of the carbene (pictured) with 91 % yield.
A copper-catalyzed asymmetric conjugate borylation (ACB) reaction was used to investigate the applicability of the CholestCAAC as a stereo-directing chiral ligand. Moderate yields (47 to 77 %) and enantioselectivities up to 55 % enantiomeric excess were reached. According to the researchers, only few classes of chiral NHCs have been used for asymmetric catalysis so far, and this is the first example of chiral CAACs in this field.
- The debut of chiral cyclic (alkyl)(amino)carbenes (CAACs) in enantioselective catalysis,
Delphine Pichon, Michele Soleilhavoup, Jennifer Morvan, Glen P. Junor, Thomas Vives, Christophe Crévisy, Vincent Lavallo, Jean-Marc Campagne, Marc Mauduit, Rodolphe Jazzar, Guy Bertrand,
Chem. Sci. 2019.
https://doi.org/10.1039/C9SC02810B