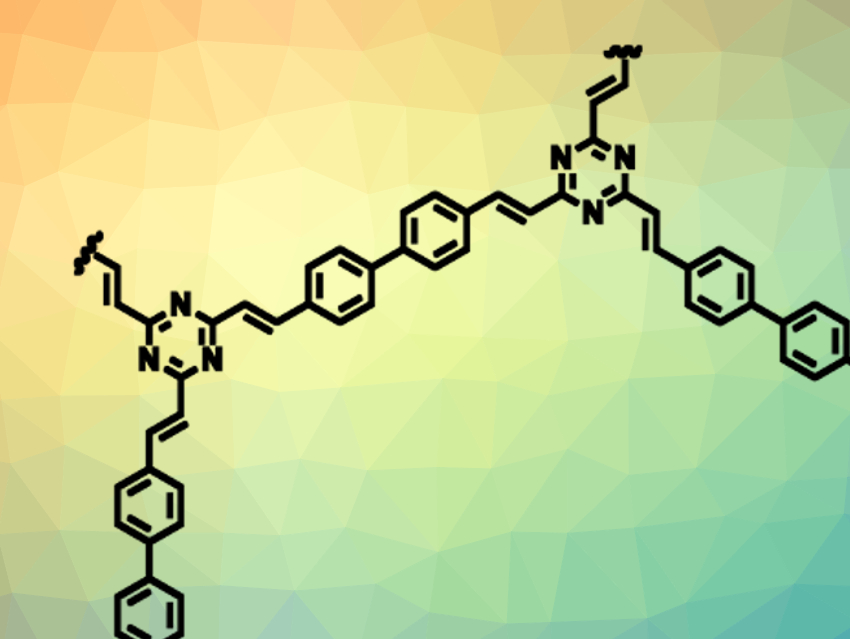
Covalent organic frameworks (COFs) consist of organic building blocks, which can form porous, crystalline, two- or three-dimensional networks. A stable link between these building blocks could, for example, be formed by a C=C bond. However, this has only been achieved so far with electron-withdrawing nitrile substituents, which weaken the link and lower the stability of the COF.
Omar M. Yaghi, Lawrence Berkeley National Laboratory and Kavli Energy NanoSciences Institute, Berkeley, CA, USA, and King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia, and colleagues have synthesized the first unsubstituted olefin-linked COF, COF-701 (section pictured). The team used 2,4,6-trimethyl-1,3,5-triazine (TMT) and 4,4′-biphenyldicarbaldehyde (BPDA) as building blocks. The substrates were linked using an aldol condensation, which was performed using trifluoroacetic acid as a catalyst at 150 °C in a mixture of mesitylene, 1,4-dioxane, and acetonitrile.
The unsubstituted olefin links give the COF high chemical stability, e.g., in the presence of strong acids, bases, or organolithium compounds. A strong Lewis acid such as BF3, for example, can be immobilized in the COF. The COF host remains stable and the Lewis acid guest retains its catalytic activity.
- Porous Crystalline Olefin-Linked Covalent Organic Frameworks,
Hao Lyu, Christian S. Diercks, Chenhui Zhu, Omar M. Yaghi,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b02848