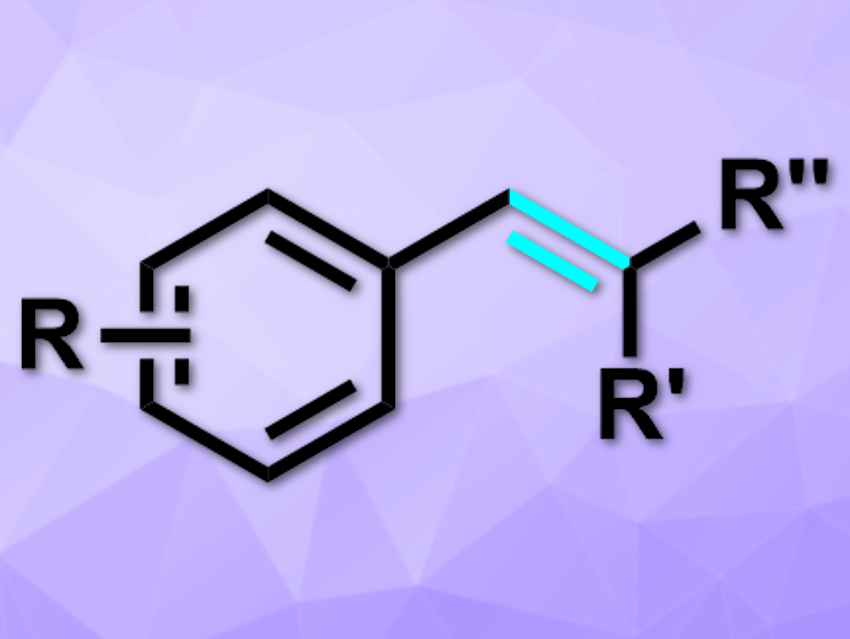
The deprotonative functionalization of C–H bonds usually requires stoichiometric amounts of strong bases. However, this approach has issues such as air- and moisture sensitivity, poor functional group tolerance, and a requirement for low temperatures.
Masanori Shigeno, Yoshinori Kondo, and colleagues, Tohoku University, Sendai, Japan, have developed a deprotonative coupling of benzylic C(sp3)–H bonds with carbonyls (example product pictured), which is catalyzed by an amide–base system formed in situ. The team used tetramethylammonium fluoride (TMAF) and tris(trimethylsilyl)amine (N(TMS)3) to create the amide base (TMS)2N–. This base can deprotonate a variety of benzylic C–H bonds. The team used different substituted arenes or heteroarenes as substrates and coupled them with a variety of ketones, non-enolizable aldehydes, or N,N-disubstituted formamides at 60 °C.
The desired products were obtained in moderate to excellent yields. The catalytic system is more effective than conventional amide bases such as potassium bis(trimethylsilyl)amide (KHMDS). The reaction works even for toluene derivatives with low reactivity.
- Catalytic Amide–Base System of TMAF and N(TMS)3 for Deprotonative Coupling of Benzylic C(sp3)–H Bonds with Carbonyls,
Masanori Shigeno, Kunihito Nakaji, Kanako Nozawa-Kumada, Yoshinori Kondo,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b00550