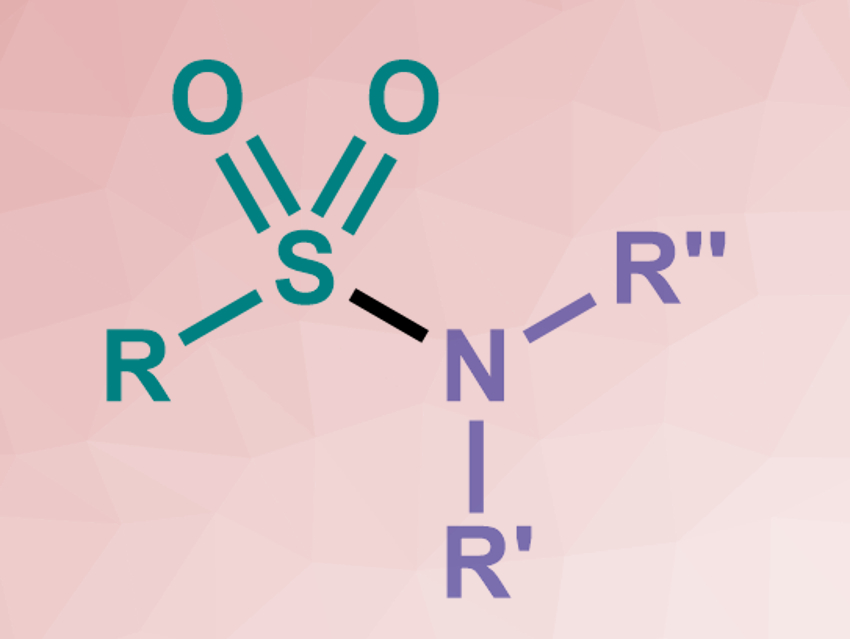
Sulfonamides (pictured) are useful compounds, e.g., in pharmaceutical or agro-chemistry. They are often synthesized from sulfonyl chlorides. However, these reagents are toxic and highly reactive. A mild, direct coupling between low-cost, readily available amines and thiols would be preferable. Developing approaches for such a reaction has been challenging.
Timothy Noël, Eindhoven University of Technology, The Netherlands, and colleagues have developed an electrochemical oxidative coupling of amines and thiols for the synthesis of sulfonamides. The team reacted ammonia, primary amines, or secondary amines with either thiols or the corresponding disulfides in an electrochemical reactor. The reaction was carried out in a mixture of acetonitrile and dilute hydrochloric acid, with Me4NBF4 as an electrolyte.
The desired products were obtained in moderate to good yields, in some cases within five minutes. Hydrogen is formed as a byproduct. The reaction does not require a catalyst or any other reagents. It has a broad substrate scope and tolerates a variety of functional groups. According to the researchers, the method could be useful in both academic and industrial settings.
- Sulfonamide Synthesis through Electrochemical Oxidative Coupling of Amines and Thiols,
Gabriele Laudadio, Efstathios Barmpoutsis, Christiane Schotten, Lisa Struik, Sebastian Govaerts, Duncan L. Browne, Timothy Noël,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b02266