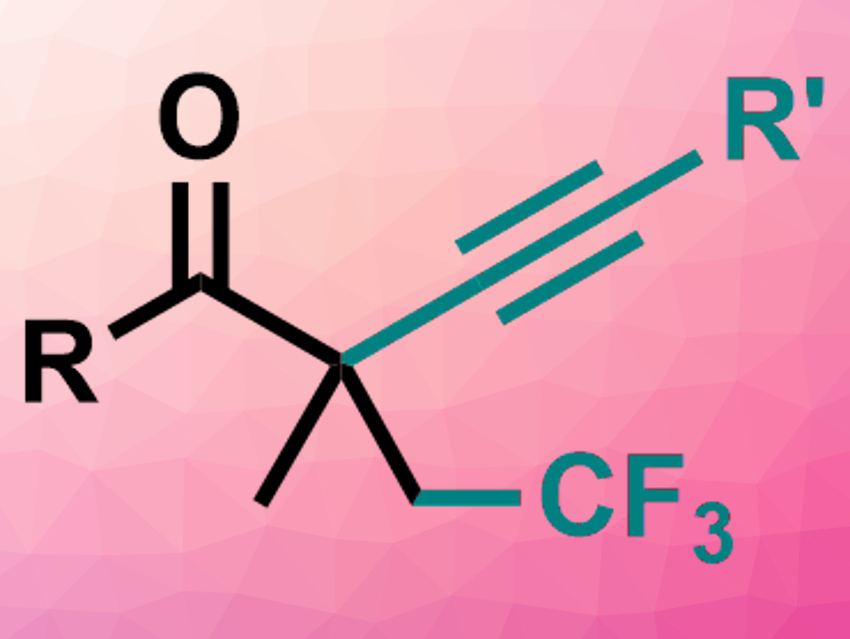
Fluorine-containing substituents are useful, for example, in medicinal, materials, and agro-chemistry. Methods for the synthesis of CF2– or CF3-containing compounds from unsaturated substrates usually require transition-metal catalysts and activated alkenes or alkynes.
Yong-Min Liang, Lanzhou University, China, and colleagues have developed a simple-to-operate radical synthesis of either difluroalkylated or trifluoromethylated alkynyl ketones from unactivated alkenes under mild conditions. For the difluoroalkylation, the team used substituted 1,4-enynes as substrates, ICF2CO2Et as a fluorination reagent. and N-methylpiperidine as a base in toluene at 140 °C. For the trifluoromethylation (example product pictured), the same substrates were reacted with CF3SO2Na as a fluorination reagent and tert-butyl hydroperoxide (TBHP) as an oxidant in acetonitrile at 80 °C.
The desired products were obtained in moderate to good yields. The reaction includes a 1,2-alkynyl migration. It proceeds through a radical pathway and does not require a metal catalyst.
- Metal-free Promoted CF2/CF3-difunctionalization of Unactivated Alkenes,
Ming Li, Xin-Yu Zhu, Yi-Feng Qiu, Ya-Ping Han, Yu Xia, Cui-Tian Wang, Xue-Song Li, Wan-Xu Wei, Yong-Min Liang,
Adv. Synth. Catal. 2019.
https://doi.org/10.1002/adsc.201900231