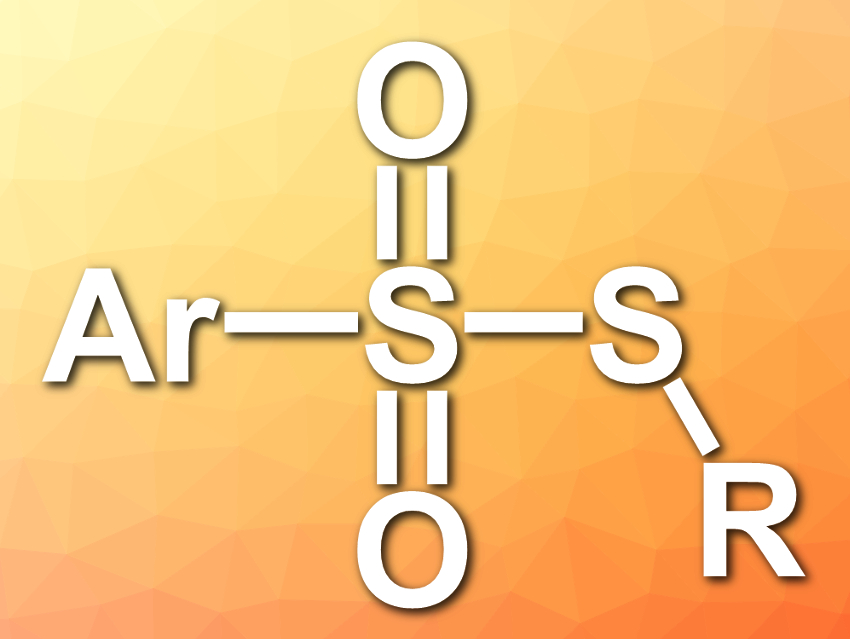
Thiosulfonates have various biological activities, e.g., antiviral, antimicrobial, antibacterial, and fungicidal activities. They can also be used as sulfenylating reagents in organic transformations.
Daoshan Yang, Qingdao University of Science and Technology and Qufu Normal University, both China, and colleagues have developed a simple, catalyst- and metal-free strategy for the synthesis of unsymmetrical thiosulfonates (pictured). The team used DABCO·(SO2)2 (DABCO = 1,4-diazabicyclo[2.2.2]octane) as a solid and bench-stable source of sulfur dioxide.
The team reacted aryldiazonium tetrafluoroborates with thiols in the presence of DABCO·(SO2)2 via an SO2 insertion to give the desired thiosulfonates in moderate to good yields. The reaction has a good functional group tolerance. The reaction can also be used for the one-pot synthesis of thiosulfonates from arylamines and thiols in the presence of tert-butyl nitrite (t-BuONO).
- Metal-Free Synthesis of Thiosulfonates via Insertion of Sulfur Dioxide,
Guoqing Li, Ziyu Gan, Kexin Kong, Xiaomeng Dou, Daoshan Yang,
Adv. Synth. Catal. 2019.
https://doi.org/10.1002/adsc.201900157