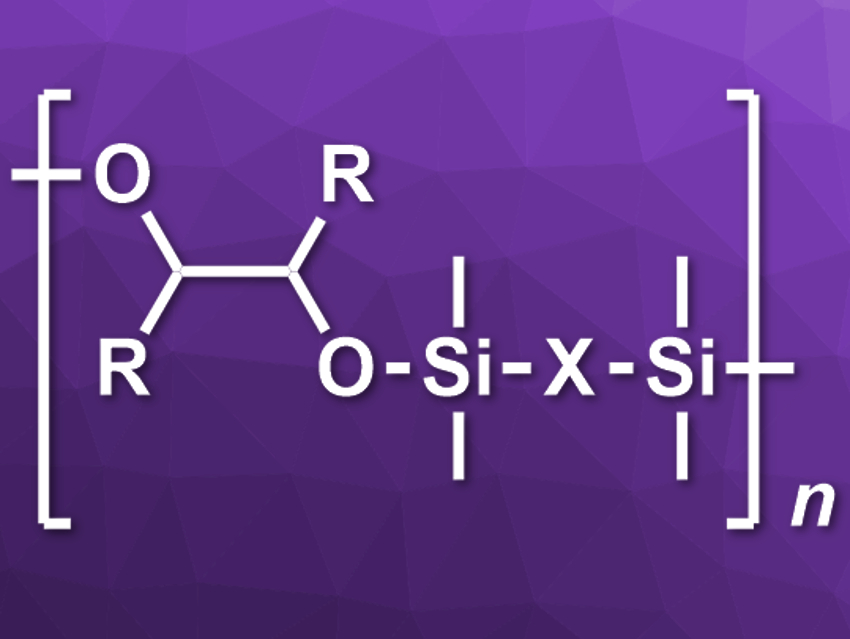
Poly(silyl ether)s (PSEs) are flexible and thermally stable high-performance materials. Existing methods for PSE synthesis either produce large amounts of byproducts or suffer from limited availability of the starting materials. The addition of silicon hydrides across unsaturated bonds could be a promising alternative, but usually requires transition-metal catalysts.
Shaoguang Li, University of California, Santa Barbara, USA, and China University of Geosciences, Wuhan, Craig J. Hawker, University of California, Santa Barbara, and colleagues have developed a protocol for the polymerization of α-diketone and bis(silane) monomers under ambient conditions via a metal-free hydrosilylation catalyzed by tris(pentafluorophenyl)borane (B(C6F5)3). The team reacted a series of readily available α-diketone and bis(silane) derivatives in the presence of 0.5 mol% B(C6F5)3 in chloroform to give the desired PSEs (pictured).
According to the researchers, the Lewis acid B(C6F5)3 activates the Si–H bond via η-coordination and promotes its addition to the ketone’s C=O bond. The thermal stability of the obtained polymers can be tuned by varying the monomers. The material’s Si–O–C backbone can be degraded under acidic conditions, which could be useful for the development of responsive polymers.
- Metal-Free Synthesis of Poly(silyl ether)s under Ambient Conditions,
Caitlin S. Sample, Sang-Ho Lee, Morgan W. Bates, Jing M. Ren, Jimmy Lawrence, Valerie Lensch, Jeffrey A. Gerbec, Christopher M. Bates, Shaoguang Li, Craig J. Hawker,
Macromolecules 2019.
https://doi.org/10.1021/acs.macromol.8b02741