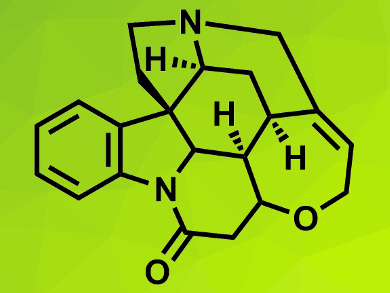
Strychnine (pictured) is a complex molecule and a challenging target for total synthesis. Chemists continually try to find more concise paths to this toxic natural product.
Yong Qin and colleagues, Sichuan University, Chengdu, China, have developed a new synthesis strategy for a concise, catalytic, asymmetric total synthesis of (+)-strychnine. The team used an enantioselective Michael addition and two cascade reactions as key steps. The synthesis started from a known chiral aldehyde ester.
A photoinduced radical cascade reaction and a bioinspired cascade rearrangement were used to construct the molecule’s framework. The photocatalytic cascade reaction allowed the team to efficiently prepare a tetracyclic alkaloid intermediate, which was converted into the desired strychnine core using an oxidation-rearrangement sequence.
- Asymmetric Total Synthesis of (+)-Strychnine,
Liping He, Xiaobei Wang, Xiaoqing Wu, Zhaoxiang Meng, Xin Peng, Xiao-Yu Liu, Yong Qin,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b03686
Also of Interest
- Strychnine: From Isolation to Total Synthesis – Part 1,
Klaus Roth
ChemViews Mag. 2015.
https://doi.org/10.1002/chemv.201500031
Just how toxic is strychnine, and why?