Late transition metal oxo complexes with d-electron counts >4 are rare. John S. Anderson and colleagues, University of Chicago, Il, USA, have used a strongly donating tris(imidazole-2-ylidene)borate scaffold to isolate two highly unusual CoIII-oxo complexes. The complexes show O and H transfer reactivity. The findings demonstrate that terminal metal oxo complexes with six d-electrons can display strong metal−oxygen bonding and sufficient stability to enable their characterization.
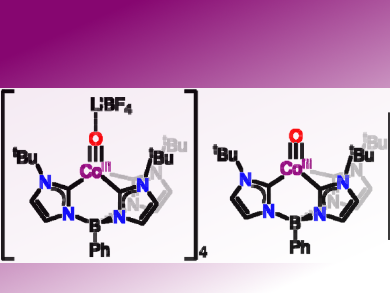
Treatment of PhB(tBuIm)3CoIICl with NaOH in THF generates the new, violet species PhB(tBuIm)3CoIIOH. Addition of one equivalent of [Fc][BF4] to this species in THF at −78 °C leads to an isosbestic conversion from the violet to a green species assigned as the one-electron oxidized product, [PhB(tBuIm)3CoIIIOH][BF4]. Treatment with strong bases such as LiHMDS (HMDS = hexamethyldisilazide) results in the generation of a new magenta species, one of the two CoIII-oxo complexes (pictured left). Addition of Kryptofix 2,2,1 (4,7,13,16,21-pentaoxa-1,10-diazabicyclo[8.8.5]tricosane, crypt) to a benzene solution of the magenta species results in the formation of a purple solution of PhB(tBuIm)3CoIIIO (pictures right) and precipitation of [Li(crypt)][BF4].
The unambiguous assignment of these complexes suggests that related species exist. According to the researchers, these studies change our understanding of the reactivity and bonding in late transition metal oxo complexes and open the door to further study the properties of this class of complexes.
- Isolation of a Terminal Co(III)-Oxo Complex,
McKenna K. Goetz, Ethan A. Hill, Alexander S. Filatov, John S. Anderson,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b07399