The plasma membrane is the primary defense mechanism for cells. This selectively permeable barrier needs to tightly regulate what gets into and out of the cell, e.g., to prevent invasions by viruses or a build-up of toxic chemicals.
Artificial channels in the cell membrane could be used for treating channelopathies (diseases caused by malfunctioning ion channels, such as cystic fibrosis) or for killing cancer cells by disrupting the ion homeostasis (equilibrium). Some success has been achieved in designing chloride carriers or channels that can self-assemble. However, thus far, they have been relatively complex molecules, which limits their use in the clinic.
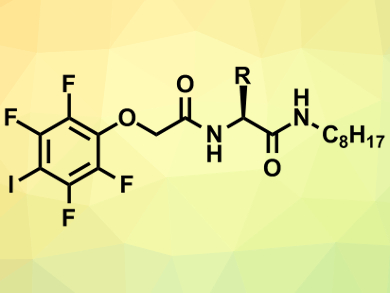
Huaqiang Zeng, Institute of Bioengineering and Nanotechnology, Singapore, and colleagues have synthesized simple monopeptide units (example pictured) that can stack to form chloride channels in vesicles. Basic experiments on model vesicles showed that the channel-forming molecules (CFMs) can selectively transport chloride ions with a gradient in exchange for hydroxide ions. Preliminary molecular modeling suggests that eight CFMs stack in the membrane, forming a chain to pass the chloride ions along.
The team did a direct comparison of their CFMs with previously discovered CFMs. They found that their simpler molecule was equally or more potent than the earlier compounds. In addition, the new CFMs were added to a culture of breast cancer cells and results showed that lethal amounts of chloride were carried into the cells and cell viability was significantly reduced. It remains to be seen if this effect will translate to a more physiologically relevant assay, but this prospect of a new way to kill cancer cells is worth exploring further.
- A halogen bond-mediated highly active artificial chloride channel with high anticancer activity,
Changliang Ren, Xin Ding, Arundhati Roy, Jie Shen, Shaoyuan Zhou, Feng Chen, Sam Fong Yau Li, Haisheng Ren, Yi Yan Yang, Huaqiang Zeng,
Chem. Sci. 2018.
https://doi.org/10.1039/c8sc00602d