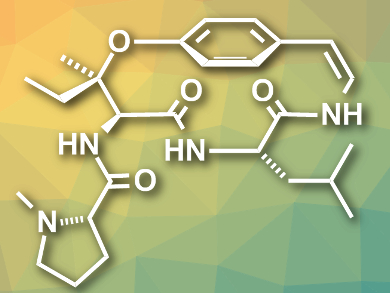
The cyclopeptide alkaloid ceanothine D (pictured) was isolated from Ceanothus americanus. This plant is also known as red root or the New Jersey tea plant, since it was used to replace imported tea during the American Revolution. A structure for ceanothine D was proposed based on 1H NMR spectroscopy, mass spectrometry (MS), and degradation studies. However, no total synthesis or comprehensive structure determination had been reported so far.
Jisun Lee and Madeleine M. Joullié, University of Pennsylvania, Philadelphia, USA, have performed the first total synthesis of the proposed structure of ceanothine D. The team started from L-leucinamide and a D-serine derivative. They used a peptide coupling, followed by an intramolecular Mitsunobu reaction, to generate an aziridine intermediate. This intermediate was coupled with a vinyl iodide, and a final macrocyclization via the opening of the aziridine ring gave the desired product in an overall yield of 8.4 %.
According to the researchers, the developed method could be useful for the synthesis of other macrocycles. It could also allow the study of the biological activity of ceanothine D and related cyclopeptide alkaloids, which are difficult to extract and purify from natural sources.
- Total Synthesis of the Reported Structure of Ceanothine D via a Novel Macrocyclization Strategy,
Jisun Lee, Madeleine M Joullie,
Chem. Sci. 2018.
https://doi.org/10.1039/c8sc00234g



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)