Catalytic and asymmetric functionalization of C(sp3)–H is a sustainable method to construct stereogenic carbon centers. Although many methods have been well established for α-C(sp3)–H functionalization of carbonyl compounds, only limited studies are known for β-C(sp3)–H funcationalization.
Eric Meggers, Pilipps-Universität Marburg, Germany, and colleagues have developed a visible-light-activated asymmetric β-C–H functionalization of 2-acyl imidazoles and 2-acylpyridines with 1,2-dicarbonyl compounds. A stereogenic-at-rhodium Lewis acid has been used as a catalyst to give the products in high yields with excellent stereoselectivities. The experiments are carried out in the presence of catalytic amount of DABCO (1,4-diazabicyclo[2.2.2]octane) under blue LED radiation in acetone at room temperature.
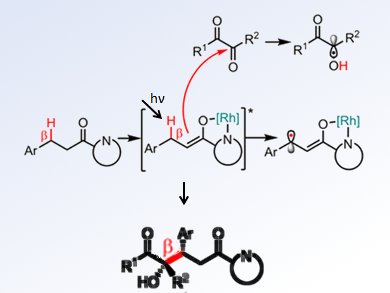
According to experimental and computational studies, the researchers proposed a mechanism in which an electron-rich Rh-enolate radical and an α-hydroxyl radical are formed and a stereocontrolled radical-radical recombination occur (pictured). They also found that the reaction strongly depended on the structure of the carbonyl compounds.
- Visible-Light-Activated Asymmetric β-C–H Functionalization of Acceptor-Substituted Ketones with 1,2-Dicarbonyl Compounds,
Jiajia Ma, Anthony R. Rosales, Xiaoqiang Huang, Klaus Harms, Radostan Riedel, Olaf Wiest, Eric Meggers,
J. Am. Chem. Soc. 2017.
DOI: 10.1021/jacs.7b09152