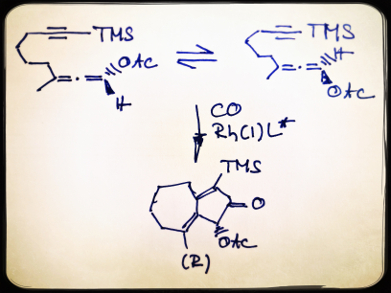
α-Oxygenated ketones and their redox derivatives are present in numerous natural products. However, enantioselective access to these compounds is limited. The rhodium-catalyzed allenic Pauson-Khand reaction (APKR) is an efficient method of synthesizing α-acyloxy cyclopentenones, but the asymmetric version using a chiral rhodium catalyst has not been developed.
Peng Liu and Kay M. Brummond, University of Pittsburgh, PA, USA, and colleagues have found an efficient chiral Rh catalyst for asymmetric APKR by a rational approach based on computational insights with experimental validation. A dibenzazepine-based chiral binaphthyl phosphoramidite is predicted as a selective and reactive ligand by computational studies and is validated by the laboratory experiments. The reactions are carried out under 0.1 atm CO in dichloroethene at 70 °C in the presence of mesitylene using Rh(cod)2BF)4 (cod = cyclooctadiene) as the catalyst.
This is the first catalyst-controlled enantioselective APKR and the first dynamic kinetic asymmetric transformation in carbonylation reaction. The researchers have successfully shown that screening a large number of chiral ligands could be avoided by using computational guided catalyst design.
- Computationally Guided Catalyst Design in the Type I Dynamic Kinetic Asymmetric Pauson–Khand Reaction of Allenyl Acetates,
Lauren C. Burrows, Luke T. Jesikiewicz, Gang Lu, Steven J. Geib, Peng Liu, Kay M. Brummond,
J. Am. Chem. Soc. 2017.
DOI: 10.1021/jacs.7b07121