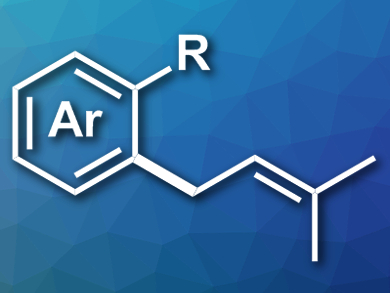
Polysubstituted allyl groups, such as the prenyl group, are useful in synthetic intermediates. They can also be found in bioactive compounds, particularly in prenylated arenes or heteroarenes. Existing processes for the synthesis of prenylated arenes can suffer from poor atom economy, harsh reaction conditions, or a need for expensive noble metal catalysts.
Honggen Wang, Sun Yat-sen University, Guangzhou, China, Qingjiang Li, Sun Yat-sen University and Peking University, Beijing, China, and colleagues have developed a manganese(I)-catalyzed coupling of arenes with allenes to introduce prenyl groups and other polysubstituted allyl groups. The team combined a variety of indoles with different polysubstituted allenes, using [MnBr(CO)5] as a catalyst, NaOAc as an additive, and 1,4-dioxane as the solvent at 100 °C. The reaction gave the desired allylated arenes (example pictured) in good to excellent yields.
The researchers were able to upscale the atom-economic synthesis to a gram scale while using a low catalyst loading (2.5 mol%). According to the team, the method could have applications in the synthesis of bioactive natural products and medicinal chemistry.
- Manganese(I)-Catalyzed Direct C–H Allylation of Arenes with Allenes,
Shi-Yong Chen, Qingjiang Li, Honggen Wang,
J. Org. Chem. 2017.
DOI: 10.1021/acs.joc.7b02220
Also of Interest
- Manganese-Catalyzed Allylation,
ChemistryViews.org 2017.
Earth-abundant metal catalysis combines C–H and C–C bond activation