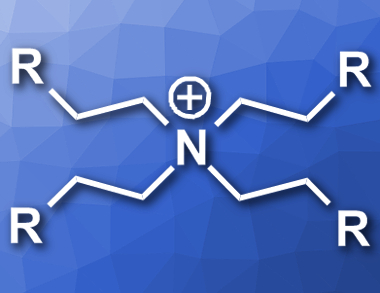
Alkylations are important reactions in organic chemistry. Activating C–H bonds and using an alkylating agent is a common approach to such conversions. The alkylating agents are often either alkyl halides, which produce stoichiometric byproducts during the reaction, or olefins, which can be gaseous and hard to handle.
Michael Schnürch, Technical University of Vienna, Austria, and colleagues have developed an alkylation reaction which uses quarternary ammonium salts (cation pictured) as alkylating agents that can replace olefins. The approach is based on a Hofmann elimination, which converts the quarternary ammonium salt into an olefin in situ. The team then tested the ammonium salts in a known rhodium(I)-catalyzed reaction protocol which usually uses olefins to alkylate benzylic amines, with KOH as a base and [RhCl(cod)2] (cod = 1,5-cyclooctadiene) as the catalyst.
The researchers found that the reaction proceeds in good yields, which drop with increasing alkyl chain length. A number of functional groups are tolerated, and the ammonium salts could even be used in conjunction with other transition metal catalysts. According to the team, ammonium salts could be broadly applicable in organic synthesis as an easy-to-handle, solid-state alternative to olefins.
- Quaternary Ammonium Salts as Alkylating Reagents in C–H Activation Chemistry,
Manuel Spettel, Robert Pollice, Michael Schnürch,
Org. Lett. 2017.
DOI: 10.1021/acs.orglett.7b01946