Nitrile reduction usually needs stoichiometric amounts of metal hydrides or hydrosilanes, which are environmentally unfriendly. The hydrogenation of nitriles has also been developed, but it suffers from several problems, e.g., a need for precious metals as catalysts, low selectivity, and limited substrate scope. The selective hydrogenation of nitriles to form secondary imines with an environmentally benign catalyst is a challenging research goal.
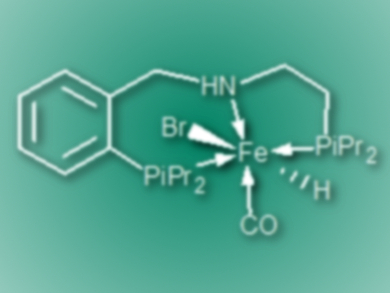
David Milstein, Weizmann Institute of Science, Rehovot, Israel, and colleagues have developed a protocol for the selective hydrogenation of a wide range of (hetero)aromatic and aliphatic nitriles to secondary imines. The team used an iron–PNP pincer complex (pictured) as the catalyst. The reactions were carried out in benzene at 90 °C under 30 bar hydrogen in the presence of the catalyst and tBuOK.
The researchers found that aromatic nitriles were generally hydrogenated in higher yields (up to 97 %) and required 1 to 4 mol % catalyst, but aliphatic nitriles gave lower yields (11 to 69 %) and required 8 mol % catalyst. In contrast to other iron PNP catalysts, no amines were observed.
- Selective Hydrogenation of Nitriles to Secondary Imines Catalyzed by an Iron Pincer Complex,
Subrata Chakraborty, David Milstein,
ACS Catal. 2017.
DOI: 10.1021/acscatal.7b00906