Polycyclic aromatic hydrocarbons (PAHs) are molecules composed of multiple aromatic rings. PAHs with a twisted structure and modified electronic features could be useful for organic electronics. Substituent-free PAHs are p-type semiconductors. Electron-withdrawing substituents can change the molecules’ energy levels and make them n-type semiconductors.
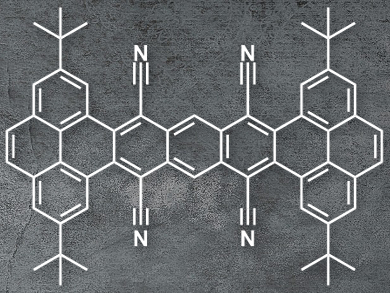
Manuel Melle-Franco, University of Aveiro, Portugal, Luis E. Hueso, CIC Nanogune, Donostia-San Sebastián, Spain, Aurelio Mateo-Alonso, University of the Basque Country, Donostia-San Sebastián, Spain, and colleagues have synthesized pyrene-fused acenes with cyano groups in key positions, which induce a twist in the molecules. The team condensed 2,7-di-tert-butylpyrene-4,5-dione with tetracyanomethylbenzene under basic conditions to give a tetracyano-substituted tetrabenzoheptacene (pictured).
The resulting product is stable under a nitrogen atmosphere up to 460 °C. There is steric hindrance between the cyano groups and the tert-butyl groups, which forces the molecule out of planarity. The twisted structure improves the compound’s solubility and changes its electronic properties. Additionally, the electron-withdrawing character of the CN groups lowers the molecules LUMO and decreases the HOMO-LUMO gap (LUMO = lowest unoccupied molecular orbital, HOMO = highest occupied molecular orbital). These properties allowed the researchers to build field-effect transistors using the substituted tetrabenzoheptacene, which showed behavior that is typical for n-type semiconductors.
- Synthesis and Properties of a Twisted and Stable Tetracyano-Substituted Tetrabenzoheptacene,
Marta Martínez-Abadía, Gabriella Antonicelli, Elisabetta Zuccatti, Ainhoa Atxabal, Manuel Melle-Franco, Luis E. Hueso, Aurelio Mateo-Alonso,
Org. Lett. 2017.
DOI: 10.1021/acs.orglett.7b00493