Metal nanoparticles (NPs) are useful heterogeneous catalysts due to their high active surface areas and easy separation from the reaction products. Oxidizing the surface of such NPs can lead to catalytically active charged metal species, e.g, Pt(IV) or Au(III).
Sung June Cho, Chonnam National University, Gwangju, Republic of Korea, Hyunwoo Kim and Hyunjoon Song, Korea Advanced Institute of Science and Technology (KAIST) and Institute for Basic Science, both Daejeon, Republic of Korea, and colleagues have used the surface oxidation of rhodium NPs to create catalytically active Rh(III) species on the particles’ surface. The team synthesized Rh NPs by the thermal decompostion of Rh(acac)3 (acac = acetylacetonate). The particles were then oxidized using N-bromosuccinimide (NBS) to form Rh(0)/Rh(III) core-shell nanoparticles.
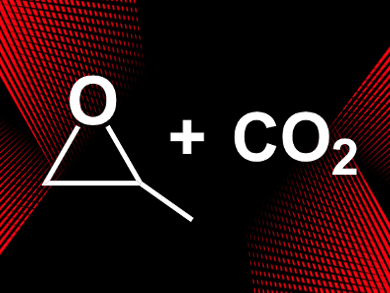
The oxidized particles can be used as Lewis acidic catalysts. The team used them for the synthesis of propylene carbonate from propylene oxide and CO2 (pictured) and found a high catalytic activity. The reaction proceeded in quantitative yield and the catalyst could be recycled five times with no change in its structure. According to the researchers, surface oxidation of other metal NPs could provide a range of new heterogeneous catalysts.
- Rh(0)/Rh(III) core–shell nanoparticles as heterogeneous catalysts for cyclic carbonate synthesis,
Younjae Jung, Taeil Shin, Kiseong Kim, Hyeeun Byun, Sung June Cho, Hyunwoo Kim, Hyunjoon Song,
Chem. Commun. 2017.
DOI: 10.1039/c6cc08318h