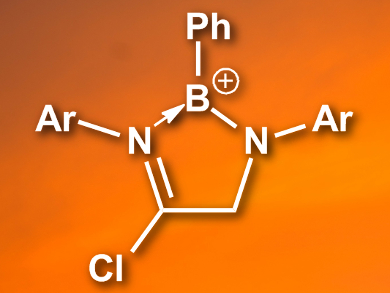
Borocations can be used in a number of reactions in organic chemistry, including hydrogenations, carboborations, haloborations, and hydroborations. Commonly, borocations are synthesized through halide or hydride abstraction from precursors which are stabilized by carbene, amine, or phophine groups.
Rebba L. Melen and colleagues, Cardiff University, UK, have developed a synthetic route to a new family of borocations (example pictured). The team prepared N,N’-1,4-diazabutadienes by a condensation between anilines and glyoxal. The diazabutadienes were reacted with dichlorophenylborane (PhBCl2) and AlCl3 to give a range of borocations in good yields. During the reaction, a chloride group was added to the starting material.
The team also prepared 4-coordinate borocations by reacting two equivalents of BCl3 with the diazabutadienes and adding AlCl3. The researchers hope that this new family of compounds can be fine-tuned to give useful borenium reagents.
- Synthesis and Reactivity of N,N’-1,4-diazabutadiene Derived Borocations,
James Lawson, Lewis Wilkins, Manon Andre, Emma Richards, Mohammed Ali, James A Platts, Rebecca Melen,
Dalton Trans. 2016.
DOI: 10.1039/c6dt03360a
Also of Interest
- Clara Immerwahr Award 2016,
ChemViews Mag. 2016.
Rebecca Melen, UK, honored for outstanding achievements in catalysis research