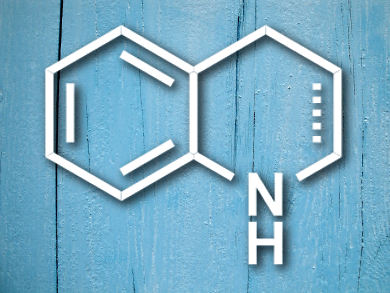
Tetrahydroquinolines (THQs) and dihydroquinolines (DHQs) (pictured) are ubiquitous in natural products, therapeutics, fluorophores, and dyes. Their synthesis has been well studied; methods range from the classic Skraup–Doebner–von Miller synthesis to catalytic and asymmetric methods.
Andrew Whiting, Durham University, UK, and colleagues have synthesized new 6-iodo-tetrahydroquinolines (THQs) and 6-iodo-dihydroquinolines (DHQs) in an easy, scalable, and practical way. The iodo-substituents are reactive substrates for direct cross-coupling. The researchers used three synthetic routes, each of which involves a different cyclization to form the heterocyclic structure.
The team prepared a versatile and stable quinolin-2-one. This could be reacted to the corresponding THQ with borane reagents. Alternatively, it could be converted to the DHQ with diisobutylaluminium hydride via a novel elimination that is more favorable at higher temperatures. Coupling the resulting strongly electron-donating scaffolds to electron-accepting moieties caused the resulting structures to exhibit strong fluorescence.
Further mechanistic insight into this reaction was gained by varying the structure of the quinolin-2-one starting material. Results indicate that increased steric bulk in the benzylic position and an N-alkyl substituent improve selectivity for the DHQ over the corresponding THQ. Currently, the team is using the optimized synthesis to produce a range of novel fluorophores.
- Practical synthetic strategies towards lipophilic 6-iodotetrahydroquinolines and -dihydroquinolines,
David R Chisholm, Garr-Layy Zhou, Ehmke Pohl, Roy Valentine, Andrew Whiting,
Beilstein J. Org. Chem. 2016, 12, 1851–1862.
DOI: 10.3762/bjoc.12.174