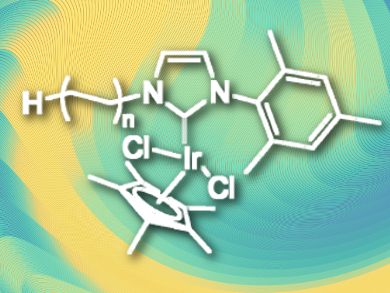
Replacing hydrogen atoms with the heavier isotope deuterium allows mechanistic investigations, is useful for mass spectrometry, and is important for metabolic and pharmacokinetics studies. Possible catalysts for this process are iridium(III) phosphine complexes, which can activate C–H bonds. However, they can decompose by ligand oxidation or dissociation, which limits their activity. N-heterocyclic carbene (NHC) ligands are more stable and show higher activity.
Christophe Boisson, Chloé Thieuleux, and colleagues, Université de Lyon, France, have developed a polyethylene-supported iridium(III)-NHC hydrogen/deuterium exchange catalyst with high activity, which can easily be recovered and reused due to its heterogeneous nature. The team prepared iodo-polyethylene (functionalized at the chain end) using catalyzed chain grow polymerization (CCG). The NHC was introduced by nucleophilic substitution of the iodide and addition of silver oxide to form Ag-NHC complexes. In a final step, a transmetallation was performed using [IrCp*Cl2]2 (Cp* = C5Me5) to give the finished PE-(NHC)IrCp*Cl2 catalyst (pictured).
The researchers tested the catalyst in a hydrogen/deuterium exchange reaction using different ketones as substrates. They found that it has the same high catalytic activity as a comparable homogeneous catalyst while being easy to reuse.
- Active and Recyclable Polyethylene-Supported Iridium-(N- Heterocyclic Carbene) Catalyst for Hydrogen/Deuterium Exchange Reactions,
Iuliia Romanenko, Sébastien Norsic, Laurent Veyre, Reine Sayah, Franck D’Agosto, Jean Raynaud, Christophe Boisson, Emmanuel Lacôte, Chloé Thieuleux,
Adv. Synth. Catal. 2016.
DOI: 10.1002/adsc.201600045