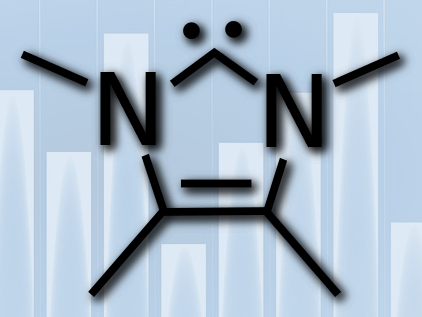
N-heterocyclic carbenes (NHCs) are one of the workhorses of modern organic chemistry. They have a wide variety of possible structures, which makes their steric and electronic properties easily tunable. They are extremely useful ligands for catalytically active transition metal complexes, for example, in olefin metathesis or cross-coupling reactions. NHCs themselves can also be used as organocatalysts.
Christopher A. Ramsden, Keele University, Staffordshire, UK, and Wojciech P. Oziminski, National Medicines Institute, Warsaw, Poland, have defined an index that allows the quantitative comparison of the ease of formation and σ-donor strength of NHCs. They used density functional theory (DFT) to calculate the energies of both the NHC and its protonated precursor molecule. The energy difference between the two is then used as the “Carbene Relative Energy of Formation (CREF) index.
The researchers calculated CREF values for a wide range of neutral and anionic five- and six-membered NHCs. They point out that, while the index is based on the cleavage of C−H bonds, it also predicts the ease of heterolytic cleavage of other σ bonds. It allows an easy prediction of the σ-donor strength of NHCs. This could help to tune the properties of NHCs for specific applications.
- Quantitative Index of the Relative Ease of Formation and σ-Bonding Strength of N-Heterocyclic Carbenes,
Christopher A. Ramsden, Wojciech P. Oziminski,
J. Org. Chem. 2016.
DOI: 10.1021/acs.joc.6b01304