Vinylcyclopropanes can be activated by transition metal catalysts such as palladium and the resulting 1,3-dipole can take part in [3+2] cycloaddtions. α,β-unsaturated aldehydes can be used as the corresponding dipolarophile. Performing these reactions in a stereoselective manner, however, has been challenging. Using a chiral organocatalyst to activate the unsaturated aldehyde could be a way to stereocontrolled reactions.
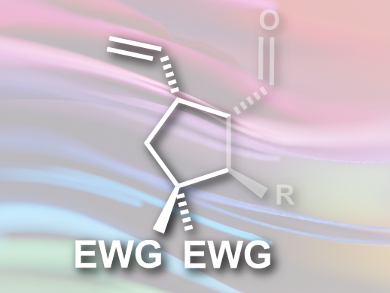
Karl Anker Jørgensen and colleagues, Aarhus University, Denmark, have developed a stereoselective [3+2] cycloaddition between vinylcyclopropanes and α,β-unsaturated aldehydes, using combined palladium- and organocatalysis. The team reacted vinylcyclopropanes containing electron-withdrawing groups, such as CN, with a range of aldehydes in the presence of Pd(dba)2 (dba = dibenzylideneacetone ) and a diphenylprolinol silyl ether as the organocatalyst. The products are substituted cyclopentanes (pictured).
The researchers propose a mechanism that proceeds via oxidative addition of the vinylcyclopropane to the palladium to form a zwitterionic π-allylpalladium intermediate, condensation between an aminocatalyst and the α,β-unsaturated aldehyde giving an iminium ion, and a final formal [3 + 2] cycloaddition between these intermediates forming the cyclopentane ring. The approach allows the stereoselective formation of substituted cyclopentanes with up to four stereocenters in good yields on a gram scale. The products can be further functionalized and have potential as useful synthetic intermediates.
- Asymmetric [3 + 2] Cycloaddition of Vinylcyclopropanes and α,β-Unsaturated Aldehydes by Synergistic Palladium and Organocatalysis,
Kim Søholm Halskov, Line Næsborg, Fernando Tur, Karl Anker Jørgensen,
Org. Lett. 2016.
DOI: 10.1021/acs.orglett.6b00852